| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 4, August 2023, pages 309-314
Retinal Vascular Density Change in Patients With Aortic Valve Regurgitation
Caner Topaloglua, c , Sinan Bilginb
aDepartment of Cardiology, Izmir University of Economics, Izmir, Turkey
bDepartment of Ophthalmology, Izmir University of Economics, Izmir, Turkey
cCorresponding Author: Caner Topaloglu, Department of Cardiology, Izmir University of Economics, Izmir, Turkey
Manuscript submitted March 29, 2023, accepted May 27, 2023, published online July 12, 2023
Short title: Retinal Vascular Density in Patients With AR
doi: https://doi.org/10.14740/cr1502
| Abstract | ▴Top |
Background: The aim of this study was to assess retinal vessel density in the superficial capillary plexus layer, deep capillary plexus layer and choriocapillaris plexus layer in patients with aortic valve regurgitation (AR) using optical coherence tomography angiography (OCTA).
Methods: Thirty-eight healthy participants (group 1) and 38 patients with AR (group 2) were assessed for this study. Diagnosis of AR is made by transthoracic echocardiography (TTE). Severity of AR was assessed according to values in the 2014 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline. Superficial capillary plexus density (SCPD), deep capillary plexus density (DCPD) and choriocapillaris plexus density (CCPD) were analyzed between groups using OCTA.
Results: SCPD measurements were found to be decreased in the nasal, inferior and central regions of patients with AR (P ≤ 0.05). DCPD measurements were found to be decreased in the nasal and inferior regions of patients with AR (P ≤ 0.05). CCPD measurements were found to be decreased in the inferior and central regions of patients with AR (P ≤ 0.05). In patients with AR, CCPD measurements were significantly decreased in the inferior region compared to the control group. Central macular thickness was found to be significantly decreased in the patients with AR.
Conclusions: Patients with AR showed decreased flow density compared with healthy controls. Retinal perfusion measured using OCTA in patients with AR may give an idea about microperfusion.
Keywords: Optical coherence tomography angiography; Aortic valve regurgitation; Microvascular changes
| Introduction | ▴Top |
Heart valve disease is defined as structural heart valves or functional impairment. Since most valve diseases are chronic and asymptomatic, epidemiological data are limited and clear figures are not known. According to Euro Heart Survey data, aortic valve regurgitation (AR) is seen in 13% [1]. Age is a significant risk factor for AR, and a 2.3 times increase in risk was detected for the past 10 years [2]. A basic diagnosis method of AR is transthoracic echocardiography (TTE). Severity of AR according to values in the 2014 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline must be decided [3]. In chronic AR, there is volume overload in the heart. Over time, it results in eccentric hypertrophy and left ventricular dilatation. The reduced flowing of blood results in the impairment of microcirculation. AR plays an important role in systemic effects on various systems, such as eye disordes, brain disorders, and cognitive disorders [4]. In AR, perfusion of not only cerebral but also other organs may be various.
Ocular blood flow is affected by various systemic diseases and metabolic conditions. Optical coherence tomography angiography (OCTA) is a noninvasive technique for evaluating ocular blood flow. It is a highly reliable, objective and repeatable imaging tool [5-7]. Evaluating retinal perfusion stations and other tissues of the human eye with OCTA presents a chance to study microcirculation in systemic disorders [8-12]. OCTA is used for assessing retinal and choroidal microvascular parameters such as vessel density, choriocapillaris flow area, foveal avascular zone (FAZ) and optic nerve head [13]. Signals of oscillating red blood cells in the choroid and retina is the principle of OCTA which show us microvascular structures in an orderly manner and help to differentiate superficial-deep flow areas and choriocapillaris flow area. OCTA analyzes not only the intensity of the reflected light but also the temporal changes of the OCT signal [14]. In neurological diseases, OCTA is frequently used and evaluated as a biomarker [15]. But retinal microcirculation in heart disease has not been evaluated widely with OCTA.
The study aimed to assess the changes in the superficial capillary plexus density, deep capillary plexus density and choriocapillaris plexus density in the retina in patients with AR.
| Materials and Methods | ▴Top |
We included 38 patients with AR who had been admitted to the Cardiology Clinic of Izmir Economy Universty. Patients with AR were categorized by 2014 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline and 2021 Valvular Heart Disease European Society of Cardiology (ESC) guideline according to clinical signs.
Thirty-eight healthy participants (mean age 48 years) were included in the study as the control group (group 1). In the study group (group 2), 38 patients with AR (mean age 52 years) were classified. All patients with AR were detected by the cardiologist. Patients with degenerative AR were included in the study. Patients in the study had normal ejection fraction and moderate or severe AR. Patients who had systemic diseases (coronary artery disease, hypertension, congestive heart failure, stroke, rheumatological diseases, diabetic disorders), aortic valve congenital anomaly (bicuspid/quadricuspid aortic valve), ascending aortic aneurysm, rheumatic mitral and tricuspid valve disease, valve stenosis (aortic, mitral or tricuspid valve), arrhythmia, smoking consumption, ophthalmologic disorders such as glaucoma, and ocular surgery history were excluded from the study. All patients and healthy individuals in the study did not use drugs related to chronic diseases. In addition, patients and healthy individuals under the age of 18 were not included in the study.
A comprehensive ophthalmic examination was performed including a slit-lamp anterior segment examination, autorefraction-keratometry, axial eye length (AL) measurement using an IOLMaster (ver.3.02; Carl Zeiss, Meditec, Jena, Germany), determination of the intraocular pressure (IOP) using Goldmann applanation tonometry, best-corrected visual acuity assessment, and a fundus examination, including indirect ophthalmoscopy, and OCTA. By using the formula SE = C/2 + S, in which C was the cylindrical power and S was the spherical power, spherical equivalent (SE) was determined.
Vascular density (VD) measurements were performed using spectral-domain OCTA (DRI OCT Tritron, Topcon, Tokyo, Japan). The OCTA machine can perform volumetric scans of the 6 × 6 mm macular area with a 320 × 320 A-scan sample density at an A-scan rate of 100,000 A-scans/s, and uses the OCTA algorithm to generate angiogram tests [16]. OCTA uses blood flow as contrast agent and provides data about VD and the flow area which is based on the split-spectrum amplitude-decorrelation angiography algorithm.
To remove saccades and minor loss of fixation, motion correction technology was performed by the software. Low-quality scans were excluded and they were repeated until good quality was achieved. In previous studies, however, a cut-off value of signal strength index was set at ≤ 40 [17]. Scans with signal strength index < 60, motion artifacts, and low-quality images because of poor fixation were excluded from the study to use more quality scans in analyses. Five areas which divided centering on macula were displayed and the blood vessel density of each area was indicated as percent. The diameter of inner circle is 1 mm, and the diameter of outside circle is displayed at 3 mm (Fig. 1a-c).
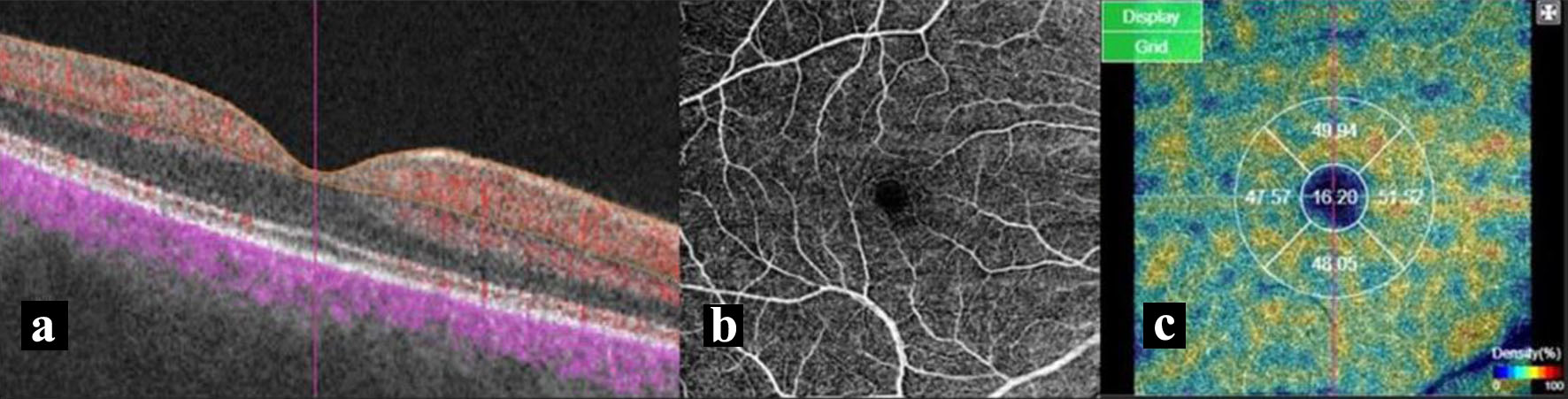 Click for large image | Figure 1. (a) Section image shows structural OCT in the background and the superficial vascular plexus data as yellow overlay. (b) Macular perfusion parameters of a 6 × 6 mm angiography scan size of superficial vascular plexus. (c) The vessel density of five areas of interest, including the fovea (1 mm diameter) and temporal, inferior, nasal, and superior quadrants. OCT: optical coherence tomography. |
Superficial capillary plexus is from 2.6 µm beneath the internal limiting membrane to 15.6 µm beneath the interface of the inner plexiform layer and inner nuclear layer. Deep capillary plexus is from 15.6 µm beneath the inner plexiform layer/inner nuclear layer to 70.2 µm beneath the inner plexiform layer/inner nuclearlayer. Choriocapillaris is from Bruch’s membrane to 10.4 µm beneath Bruch’s membrane [18]. Wave length and light source differences can lead to variable detection of choroidal vasculature in different devices [19].
The Ethics Committee of the University of IEU Medical Park University approved this prospective study. The study was conducted in compliance with the institution’s ethical standards and the revised Declaration of Helsinki.
Statistical analysis
Statistical analyses were performed with IBM SPSS for Windows Version 25.0 software. Numerical variables were expressed as mean ± standard deviation (SD). Categorical variables were summarized as numbers and percentages. The normality of the distribution and variance homogeneity of continuous variables was evaluated with the aid of the Levene’s test. Independent samples t-test was used for data with normal distribution, and Mann-Whitney U test was used for data not with normal distribution. P value of 0.05 was considered to reflect statistical significance.
| Results | ▴Top |
Each of the control (group 1) and patient groups (group 2) included 38 patients. The mean age of the patients was 48 years in group 1 and 52 years in group 2. Of the 38 participants in both groups, 17 were female and 21 were male. There was no statistically significant difference in body mass index (BMI) between the groups. In addition, no statistically significant difference was found between fasting blood glucose, creatinine, low-density lipoprotein (LDL), high-density lipoprotein (HDL), thyroid-stimulating hormone (TSH) and hemoglobin levels. Systolic and diastolic blood pressures were 122.65/78.26 mm Hg in the control group and 142.34/74.03 mm Hg in group 2 (P ≤ 0.05).
There was no statistically significant difference between the left ventricular ejection fractions of both groups (group 1: female 60.80±3.45%, male 61.10±3.81% and group 2: female 59.64±5.85%, male 60.43±6.20%). Of the 17 female patients diagnosed with AR by TTE, 12 (31.6%) had moderate AR and five (13.2%) had severe AR. In addition, of the 21 male patients, 15 (39.5%) had moderate and six (15.7%) had severe AR. When the left ventricle end systolic diameter in group 2 (female 39.64 ± 1.99 mm, male 39.68 ± 1.71 mm) was compared with the control group (female 38.93 ± 2.10 mm, male 39.75 ± 1.91 mm), no statistically significant difference was found. However, when the left ventricular end diastolic diameter was compared with the control group (female 49.11 ± 1.90 mm, male 50.55 ± 2.15 mm), a statistically significant difference was found in group 2 (female 53.47 ± 2.52 mm, male 54.50 ± 2.90 mm, P ≤ 0.05).
IOP was 16.05 mm Hg in group 1 and 14.55 mm Hg in group 2. The SE and AL were -0.71, 23.55 mm in group 1 and -0.49, 23.35 mm in group 2, respectively. Central macular thickness (CMT) was found to be 242.78 µm in group 1 and 229.50 µm in group 2, and it was found to be significantly lower in patients with AR (P ≤ 0.05) (Table 1).
 Click to view | Table 1. Demographic, Clinical, Laboratory and Echocardiographic Characteristics of Controls and Patients With AR |
SCPD) was found to be 46.47 in the temporal region, 45.39 in the nasal region, 48.99 in the superior region, 48.91 in the inferior region, and 18.78 in the central region. In group 2, it was determined as 45.13 in the temporal region, 43.64 in the nasal region, 48.74 in the superior region, 45.27 in the inferior region, and 17.00 in the central region. In SCPD measurements, it was statistically significant that it was decreased in the nasal, inferior and central regions of patients with AR (P ≤ 0.05).
In group 1, deep capillary plexus density (DCPD) was found to be 46.21 in the temporal region, 47.39 in the nasal region, 48.66 in the superior region, 50.61 in the inferior region, and 17.89 in the central region. In group 2, it was determined as 45.32 in the temporal region, 45.44 in the nasal region, 49.77 in the superior region, 47.08 in the inferior region, and 17.40 in the central region. In DCPD measurements, it was statistically significant that it was decreased in the nasal and inferior regions of patients with AR (P ≤ 0.05).
In group 1, choriocapillaris plexus density (CCPD) was found to be 54.30 in the temporal region, 54.04 in the nasal region, 53.00 in the superior region, 54.48 in the inferior region, and 55.00 in the central region. In group 2, it was determined as 54.31 in the temporal region, 53.87 in the nasal region, 52.23 in the superior region, 53.05 in the inferior region, and 52.69 in the central region. In CCPD measurements, it was statistically significant that it was decreased in the inferior and central regions of patients with AR (P ≤ 0.05).
Unlike the decrease of the central SCPD and CCPD measurements in patients with AR, the decrease of the central DCPD measurements was not found to be statistically significant. In addition, different from the decrease of nasal SCPD and DCPD measurements in patients with AR, the decrease of nasal CCPD measurements was not statistically significant.
In patients with AR, it was determined that CCPD measured from the inferior regions was significantly decreased compared to the control group (Table 2).
 Click to view | Table 2. SCPD, DCPD, CCPD and CMT Measurements |
| Discussion | ▴Top |
The retina is one of the end organs. Retinal vessels are easily accessible compared with other end organs offering direct access for evaluation of the microvasculature [20]. An OCTA evaluation is easy to perform, objective, and complication-free. Also OCTA is a promising biomarker supporting diagnosis and providing prognostic information [21]. Easily assessed retinal microvascularity is an indicator of systemic circulation. Reductions in retinal VD have been demonstrated in patients with congenital heart disease and coronary artery disease [22]. Decreased VD in diabetic retinopathy is associated with decreased visual function [23]. Reduction in the retinal blood supply to inner retinal neurons may cause cell death and ischemia [24].
In the EYE-Myocardial Infarction (EYE-MI) study involving 237 patients, retinal VD measurements were found to be associated with cardiovascular risk. Moreover, low retinal VD was also found in patients with systolic heart failure. The EYE-MI study demonstrated the association between retinal VD reduction and high-risk cardiovascular disease [25]. Retinal vessel density and choroidal vessel density have been shown to decrease in coronary artery disease [26]. Retinal vessel density, decrease in retinal and choroidal thicknesses are indicators of coronary artery disease [27]. On the other hand, a significant decrease in retinal VD has also been demonstrated in patients with hypertension [28].
It is known that changes in left ventricular volume and/or pressure due to heart valve diseases affect coronary microvascular hemodynamics. AR causes an increase in volume and eccentric hypertrophy in the heart. This situation, which changes the structure of the heart, affects the coronary microcirculation [29]. However, there is very limited information in the literature regarding the effects of eye microcirculation.
AR is common in rheumatological diseases [30]. Severe retinal microvascular changes have been demonstrated in patients with ankylosing spondylitis [31].
Doppler optical coherence tomography system has been shown to enable the measurement of retinal microcirculation in patients with AR with Takayasu arteritis. Doppler optical coherence tomography showed the reflection of the changes in the circulatory system to the retina [32].
In patients with Marfan syndrome, there may be insufficiency of the heart valve due to heart valve disorders. Thus, vessel wall compliance may be reduced and structural modification of the arterial wall may be induced. Aneurysm formation has been reported in Marfan syndrome, and this disorder also appears in small arteries. Thus, microvascular changes can occur in the vessel wall [33]. In patients with Marfan syndrome, superficial vascular plexus density was decreased [34].
In our study, we evaluated retinal plexus vessel density in more detail as superficial, deep and choriocapillaris. We found that in patients with AR, the SCPD (nasal, inferior and central regions) was significantly reduced compared to the healthy controls. In addition, in patients with AR, the DCPD (nasal and inferior regions) and CCPD (inferior and central regions) were significantly reduced compared to the healthy controls. In our study, decrease of the CMT was found in patients with AR.
The limitations of this study are single-center study, small sample size and a lack of long-term follow-up.
Conclusion
Standard TTE is the superior method for diagnosing AR. Retinal microvascular changes can be seen in patients diagnosed with moderate and severe AR by TTE, and OCTA is a promising imaging modality for detecting retinal microvascular changes in these patients. It can be used for the diagnostics and quantification of retinal microvascular changes. In addition, AR should be investigated in patients with retinal microvascular changes by OCTA. Retinal microvascular changes can be an early sign for AR with patients. Detection of early stage microvascular disorders may indicate a high risk for the development of cardiovascular disease. Generally in our study, retinal microvascular changes are observed in patients with moderate to severe AR and OCTA detects these changes but further studies are needed.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
There is no conflict of interest between the authors.
Informed Consent
All participants or their representatives provided written informed consent.
Author Contributions
Caner Topaloglu: data collection, investigation, writing (original draft, review and editing), statistical analysis, methodology, manuscript revision, supervision, final approval, project administration. Sinan Bilgin: data collection, investigation. All authors reviewed this study and approved the content of the article.
Data Availability
The data supporting these findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231-1243.
doi pubmed - Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83(6):897-902.
doi pubmed - Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O'Gara PT, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57-185.
doi pubmed - Saha M, Muppala MR, Castaldo JE, Gee W, Reed JF, 3rd, Morris DL. The impact of cardiac index on cerebral hemodynamics. Stroke. 1993;24(11):1686-1690.
doi pubmed - Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181.
doi pubmed pmc - Drexler W, Sattmann H, Hermann B, Ko TH, Stur M, Unterhuber A, Scholda C, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121(5):695-706.
doi pubmed - Fercher AF. Optical coherence tomography - development, principles, applications. Z Med Phys. 2010;20(4):251-276.
doi pubmed - Lange PS, Lahme L, Esser E, Frommeyer G, Fischer AJ, Bode N, Howel D, et al. Reduced flow density in patients with atrial fibrillation measured using optical coherence tomography angiography. Acta Ophthalmol. 2020;98(6):e789-e790.
doi pubmed - Alkan AA, Uslu Dogan C, Turker IC. Optical coherence tomography angiography for evaluation of retinal vascular changes in patients with psoriasis according to disease severity. Ocul Immunol Inflamm. 2022;30(2):433-438.
doi pubmed - Ava S, Erdem S, Karahan M, Dursun ME, Hazar L, Sen HS, Keklikci U. Evaluation of the effect of obstructive sleep apnea syndrome on retinal microvascularity by optical coherence tomography angiography. Photodiagnosis Photodyn Ther. 2022;38:102761.
doi pubmed - Kurtul BE, Cakmak AI, Kasapoglu Dilek E, Dikmen N. Evaluation of retinal microvasculature according to stable chronic obstructive pulmonary disease severity and the correlation of pulmonary parameters with optical coherence tomography angiography findings. Indian J Ophthalmol. 2022;70(5):1669-1677.
doi pubmed pmc - Sonmez HK, Arda H, Gulmez Sevim D. Evaluation of optic disc perfusion with optical coherence tomography angiography in acute non-arteritic anterior ischemic optic neuropathy. Turk J Ophthalmol. 2022;52(1):30-36.
doi pubmed pmc - Hua D, Xu Y, Zeng X, Yang N, Jiang M, Zhang X, Yang J, et al. Use of optical coherence tomography angiography for assessment of microvascular changes in the macula and optic nerve head in hypertensive patients without hypertensive retinopathy. Microvasc Res. 2020;129:103969.
doi pubmed - Rocholz R, Corvi F, Weichsel J, Schmidt S, Staurenghi G. OCT Angiography (OCTA) in Retinal Diagnostics. In: Bille JF, ed. High resolution imaging in microscopy and ophthalmology: new frontiers in biomedical optics. Cham (CH). 2019; p. 135-160.
doi pubmed pmc - Augustin AJ, Atorf J. The value of optical coherence tomography angiography (OCT-A) in neurological diseases. Diagnostics (Basel). 2022;12(2):468.
doi pubmed pmc - Lu Y, Wang JC, Zeng R, Katz R, Vavvas DG, Miller JW, Miller JB. Quantitative comparison of microvascular metrics on three optical coherence tomography angiography devices in chorioretinal disease. Clin Ophthalmol. 2019;13:2063-2069.
doi pubmed pmc - Wang Q, Chan S, Yang JY, You B, Wang YX, Jonas JB, Wei WB. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol. 2016;168:95-109.
doi pubmed - Gokmen O, Ozgur G. The effect of religious fasting and dehydration at Ramadan on choroidal thickness and Retinal vessel densities, measured with optical coherence tomography angiography. Eur J Ophthalmol. 2021;31(2):497-504.
doi pubmed - Miller AR, Roisman L, Zhang Q, Zheng F, Rafael de Oliveira Dias J, Yehoshua Z, Schaal KB, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2017;58(3):1499-1505.
doi pubmed pmc - DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
pubmed - Coscas F, Sellam A, Glacet-Bernard A, Jung C, Goudot M, Miere A, Souied EH. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT211-223.
doi pubmed - Li C, Zhong P, Yuan H, Dong X, Peng Q, Huang M, Wu Q, et al. Retinal microvasculature impairment in patients with congenital heart disease investigated by optical coherence tomography angiography. Clin Exp Ophthalmol. 2020;48(9):1219-1228.
doi pubmed pmc - Lee MW, Lee WH, Ryu CK, Kim TY, Lim HB, Lee YH, Kim JY. Effects of prolonged type 2 diabetes on the inner retinal layer and macular microvasculature: an optical coherence tomography angiography study. J Clin Med. 2020;9(6):1849.
doi pubmed pmc - Trinh M, Kalloniatis M, Nivison-Smith L. Vascular changes in intermediate age-related macular degeneration quantified using optical coherence tomography angiography. Transl Vis Sci Technol. 2019;8(4):20.
doi pubmed pmc - Arnould L, Guenancia C, Azemar A, Alan G, Pitois S, Bichat F, Zeller M, et al. The EYE-MI pilot study: a prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59(10):4299-4306.
doi pubmed - Wang J, Jiang J, Zhang Y, Qian YW, Zhang JF, Wang ZL. Retinal and choroidal vascular changes in coronary heart disease: an optical coherence tomography angiography study. Biomed Opt Express. 2019;10(4):1532-1544.
doi pubmed pmc - Matuleviciute I, Sidaraite A, Tatarunas V, Veikutiene A, Dobiliene O, Zaliuniene D. Retinal and choroidal thinning-a predictor of coronary artery occlusion? Diagnostics (Basel). 2022;12(8):2016.
doi pubmed pmc - Zeng R, Garg I, Bannai D, Kasetty M, Katz R, Park JY, Lizano P, et al. Retinal microvasculature and vasoreactivity changes in hypertension using optical coherence tomography-angiography. Graefes Arch Clin Exp Ophthalmol. 2022;260(11):3505-3515.
doi pubmed - Nishi T, Kitahara H, Saito Y, Nishi T, Nakayama T, Fujimoto Y, Matsumiya G, et al. Invasive assessment of microvascular function in patients with valvular heart disease. Coron Artery Dis. 2018;29(3):223-229.
doi pubmed - Choi E, Mathews LM, Paik J, Corretti MC, Wu KC, Michos ED, Hays AG, et al. Multimodality evaluation of aortic insufficiency and aortitis in rheumatologic diseases. Front Cardiovasc Med. 2022;9:874242.
doi pubmed pmc - van Bentum RE, Baniaamam M, Kinaci-Tas B, van de Kreeke JA, Kocyigit M, Tomassen J, den Braber A, et al. Microvascular changes of the retina in ankylosing spondylitis, and the association with cardiovascular disease - the eye for a heart study. Semin Arthritis Rheum. 2020;50(6):1535-1541.
doi pubmed - Tani T, Takahashi A, Nagaoka T, Yoshida A. Abnormality of retinal arterial velocity profiles using Doppler Fourier-domain optical coherence tomography in a case of Takayasu's arteritis with aortic regurgitation. Am J Ophthalmol Case Rep. 2017;5:134-136.
doi pubmed pmc - Awais M, Williams DM, Deeb GM, Shea MJ. Aneurysms of medium-sized arteries in Marfan syndrome. Ann Vasc Surg. 2013;27(8):1188.e1185-1187.
doi pubmed - Rezar-Dreindl S, Eibenberger K, Told R, Unterluggauer V, Sacu S, Schmidt-Erfurth U, Stifter E. Microvascular retinal changes in patients with marfan syndrome. Curr Eye Res. 2022;47(8):1186-1192.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


