| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Case Report
Volume 14, Number 1, February 2023, pages 79-85
An Uncommon Case of Atrial Fibrillation due to a Lung Mass Invasion of the Left Atrial Cavity
Ali Rahmana, Sura Alqaisia, Shiv Krishnaswamya, Emilio Hospedalesb, Walif Ajic
aInternal Medicine, Memorial Hospital West, Pembroke Pines, FL 33028, USA
bFAU Charles E. Schmidt College of Medicine, Boca Raton, FL, USA
cMemorial Cardiac and Vascular Institute, Hollywood, FL, USA
dCorresponding Author: Ali Rahman, Internal Medicine, Memorial Hospital West, Pembroke Pines, FL 33028, USA
Manuscript submitted January 31, 2023, accepted February 11, 2023, published online February 25, 2023
Short title: Uncommon AF due to Lung Mass Invasion
doi: https://doi.org/10.14740/cr1473
| Abstract | ▴Top |
Atrial fibrillation remains one of the most common conditions that clinical physicians encounter on a daily basis in the inpatient setting. This arrhythmia brings with it numerous complications if not treated properly and leads to intensive analysis of its primary etiology which is unique to every patient. In this case, we present a previously asymptomatic individual who presented to the hospital with respiratory complaints and was found to have a large lung mass, consistent with neuroendocrine lung cancer with direct compression of the left atrium leading to new-onset atrial fibrillation.
Keywords: Atrial fibrillation; Neuroendocrine cancer; Lung cancer
| Introduction | ▴Top |
Atrial fibrillation (AF) remains the most commonly encountered cardiac arrhythmia by practicing clinicians in the United States. It can be classified under different forms: paroxysmal, persistent, long-standing, and permanent. It can also be categorized by its presenting symptoms, subclinical/occult AF, or if it is associated with any cardiac valvular abnormalities, valvular AF. Regardless of classification, AF has been proven to pose great amount of risk to overall patient outcomes primarily with regards to heart failure (HF), venous thromboembolism (VTE), and death [1]. The prevalence and risk factors have been well studied and provided clinicians with important tools to assess potential risk. The ATRIA study concluded that age was one of the most important factors for developing AF showing that out of 1.9 million subjects with AF, 70% of individuals were at least 65 years old and 45% were at least 75 years old [2]. It has been shown that AF is more common in men than women as well as predominantly affecting White individuals compared to demographically matched African American, Asian, and Hispanic populations.
There are many well-known cardiac risk factors for AF including hypertensive heart disease, coronary artery disease (CAD), valvular heart disease (primarily mitral stenosis), and HF. Other common non-cardiac risk factors for the development of AF are obstructive sleep apnea, obesity, hyperthyroidism, and chronic kidney disease (CKD) [3]. Genetic factors have also been shown to play a significant role in the risk of developing AF independent of demographics such as sex and age. In addition, many patients develop AF as a result of polypharmacy (especially with non-steroidal anti-inflammatory drugs (NSAIDs), and bisphosphonates) or excess alcohol and caffeine intake [4].
In this case report, we discuss a 66-year-old gentleman with no prior structural cardiac or pulmonary conditions who presented to the emergency department (ED) with the chief complaint of dyspnea on exertion and was found to have a large malignant neoplasm of the lung which led to development of new-onset AF from direct involvement with the left atrium. Despite the myriad of risk factors of AF that have been studied and presented previously, there are only a handful of rare cases of new-onset AF caused directly by malignant neoplasms in previously asymptomatic patients. From this case report, we reiterate that regardless of the etiology for a patient’s AF, it is most important to come to a quick and efficient diagnosis which leads to more positive patient outcomes.
| Case Report | ▴Top |
Our patient was a 66-year-old man with a past medical history of hypertension (HTN), depression, and chronic back pain who initially presented to the ED with shortness of breath on exertion first noticed at work, lightheadedness, headache, and back pain between his shoulder blades. The patient reported an unintentional 10-pound weight loss over the past 4 months. He has also experienced a productive cough with white phlegm that has worsened his dyspnea. Social history was significant for smoking at least 1 pack of cigarettes daily since high school, approximately 50 pack years. He denied any alcohol or illicit drug use. The patient denied fever, chills, chest pain, palpitations, diaphoresis, sore throat, hemoptysis, nausea, vomiting, abdominal pain, dysuria, or decreased range of motion of his upper extremities. His only home medication was lisinopril 10 mg daily for HTN. He denied any allergies, recent travel history within the last 2 months, or exposure to sick individuals.
His vitals on admission included blood pressure 111/56, heart rate 65, temperature 38 °C, respiratory rate 23, and oxygen saturation 98% on room air. The patient was a normal appearing man in no acute distress. Mouth exam showed moist mucosa without lesions or erythema. His oropharynx was clear. Cardiac examination revealed normal S1/S2 heart sounds without jugular venous distention, murmurs, or friction rub. There was no peripheral edema noted. Lung exam revealed appropriate respiratory effort with course breath sounds. No wheezing, crackles, or rhonchi were appreciated. Musculoskeletal and neurological exams were unremarkable.
Initial completed blood count showed white blood cells 10.8 × 103/µL, hemoglobin 8.3 g/dL, and platelets 490 × 103/µL. The patient’s initial complete metabolic panel was only remarkable for a creatinine of 1.24 mg/dL and alkaline phosphatase of 133 U/L. Cardiac and pulmonary labs included negative troponin (× 3), elevated proB-type natriuretic peptide (proBNP) of 1,410 pg/mL, and elevated D-dimer of 1.89 mg/L fibrinogen equivalent unit (FEU). Thyroid panel revealed mildly decreased thyroid-stimulating hormone (TSH) of 0.409 IU/mL with a normal T4. coronavirus disease 2019 (COVID-19), influenza, and respiratory syncytial virus (RSV) panel were negative. His initial electrocardiogram (ECG, Fig. 1) showed AF with rapid ventricular rate (RVR) at a rate of 143 beats/min.
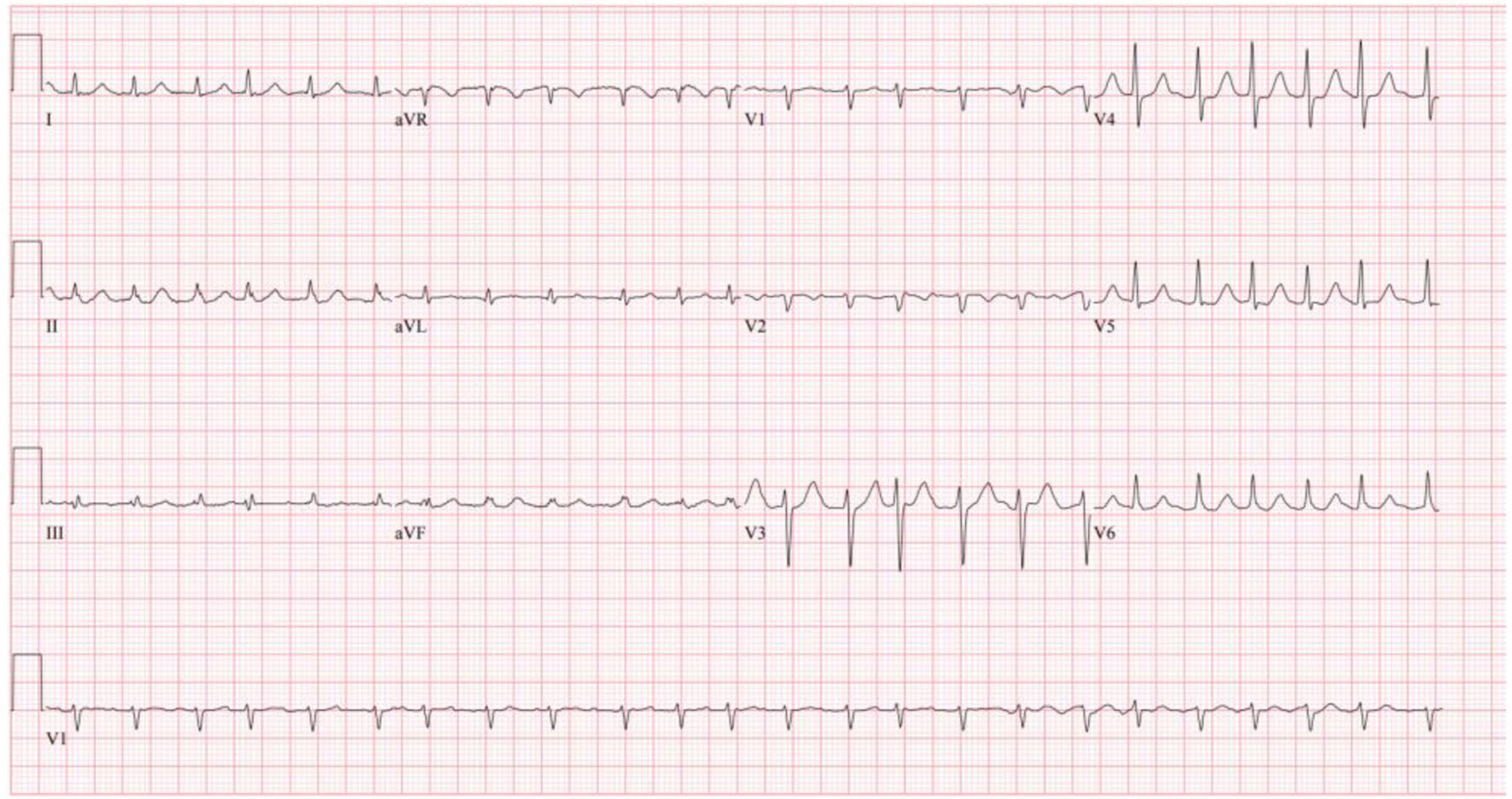 Click for large image | Figure 1. Patient’s initial ECG on admission showing atrial fibrillation with RVR. ECG: electrocardiogram; RVR: rapid ventricular rate. |
He was started on 15 mg of diltiazem and given a second dose 27 min later. A repeat ECG 2 h after diltiazem administration (Fig. 2) showed resolution of the AF with RVR and return to normal sinus rhythm.
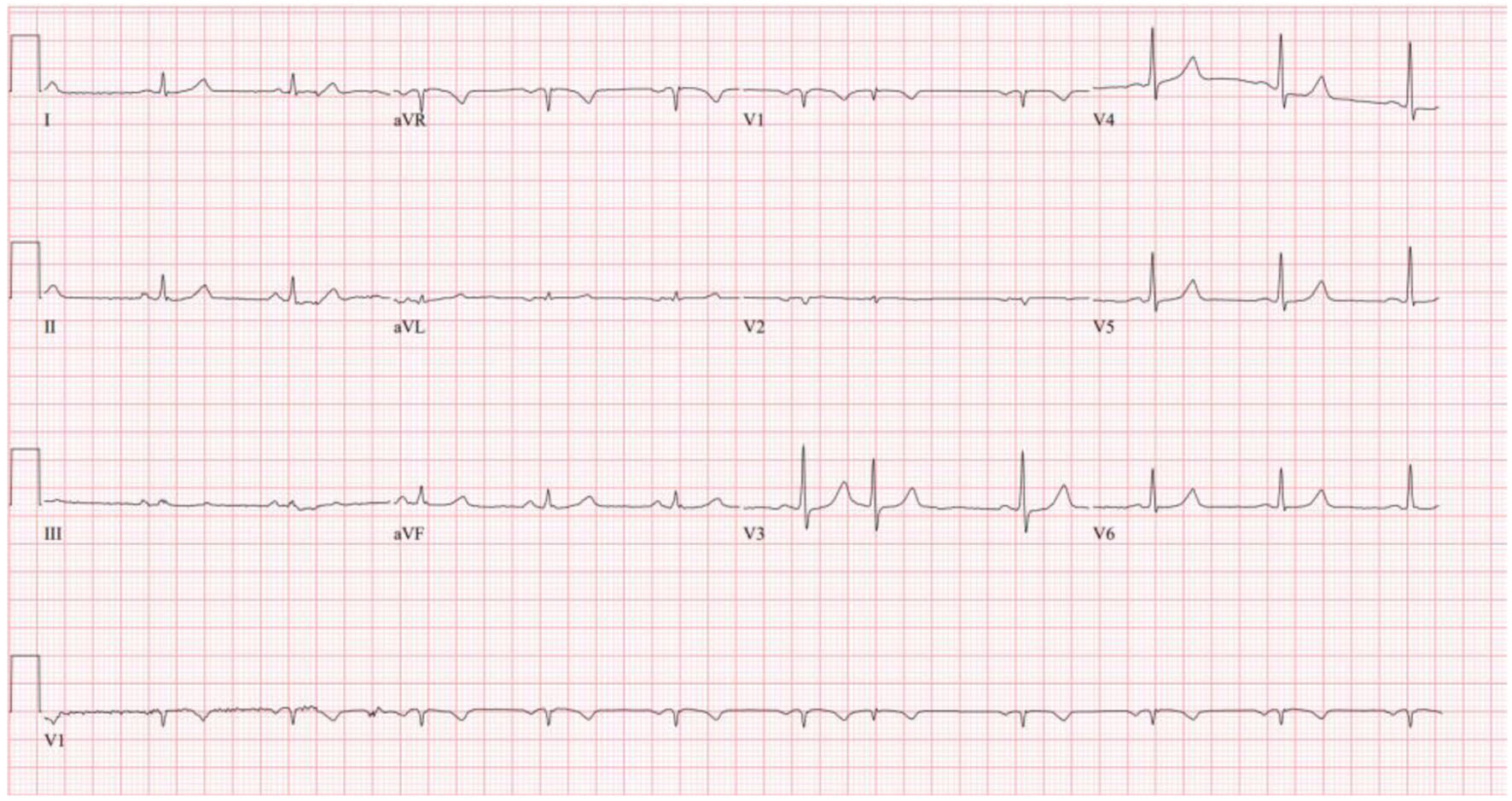 Click for large image | Figure 2. Repeat ECG 2 h after diltiazem administration showing return to normal sinus rhythm. ECG: electrocardiogram. |
Chest X-ray (Fig. 3) showed a 9.3 cm right perihilar mass. The patient’s Padua score, which represents the risk of VTE, was 6 which represents an 11% risk of symptomatic VTE. Therefore, a computed tomography pulmonary angiography (CTPA) with intravenous (IV) contrast (Figs. 4-6) was performed and illustrated a 1.91 × 2.11 cm right hilar lymph node, 1.5 × 1.04 cm mediastinal lymph node anterior to the trachea, and a 9.57 × 7.97 cm right lower lobe mass, highly suspicious for malignancy. There was also noted to be mild bilateral pleural effusions and hyperinflation of the lungs, consistent with chronic obstructive pulmonary disease (COPD)/emphysema.
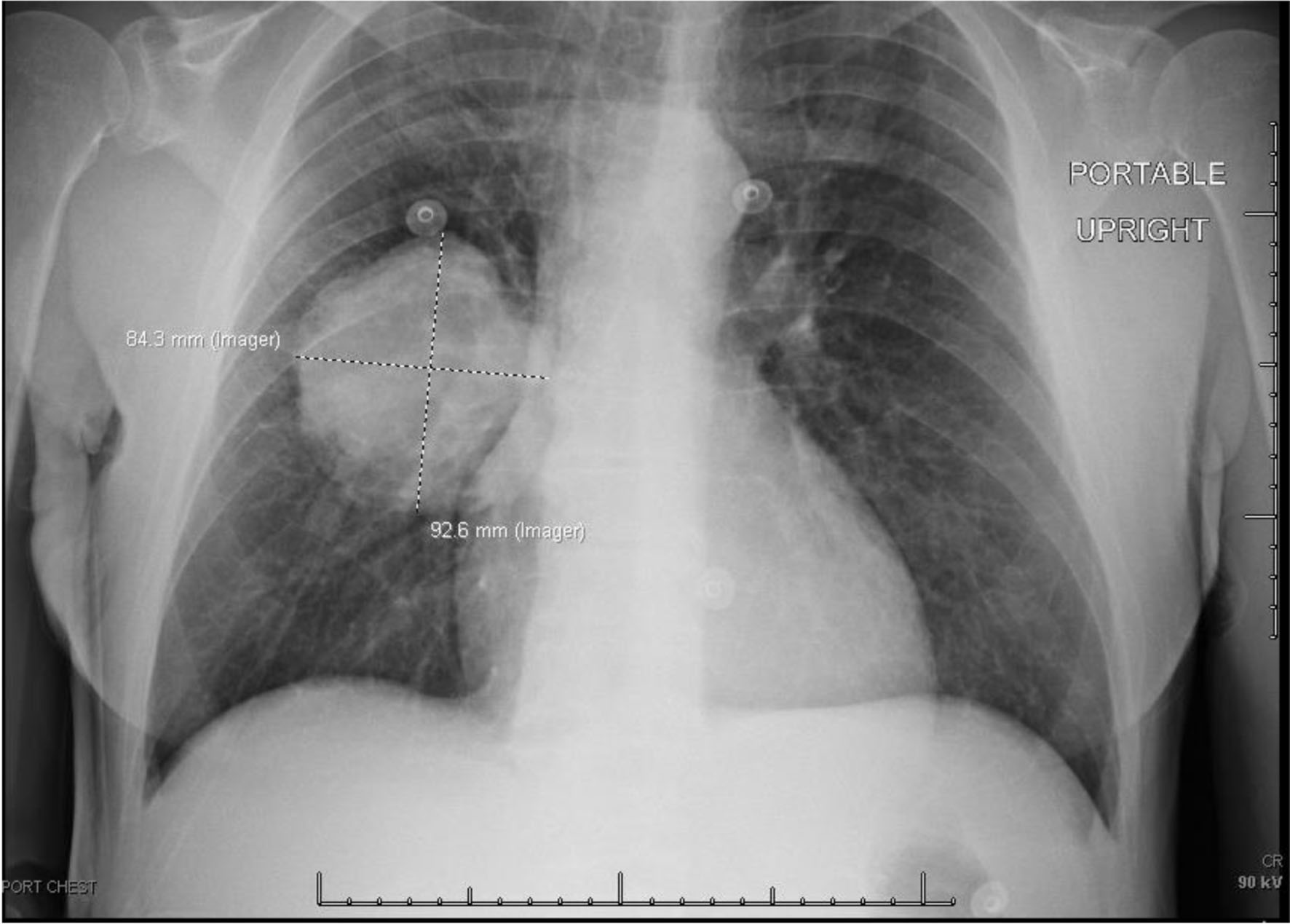 Click for large image | Figure 3. Chest X-ray on admission showing large centrally located right lung mass. |
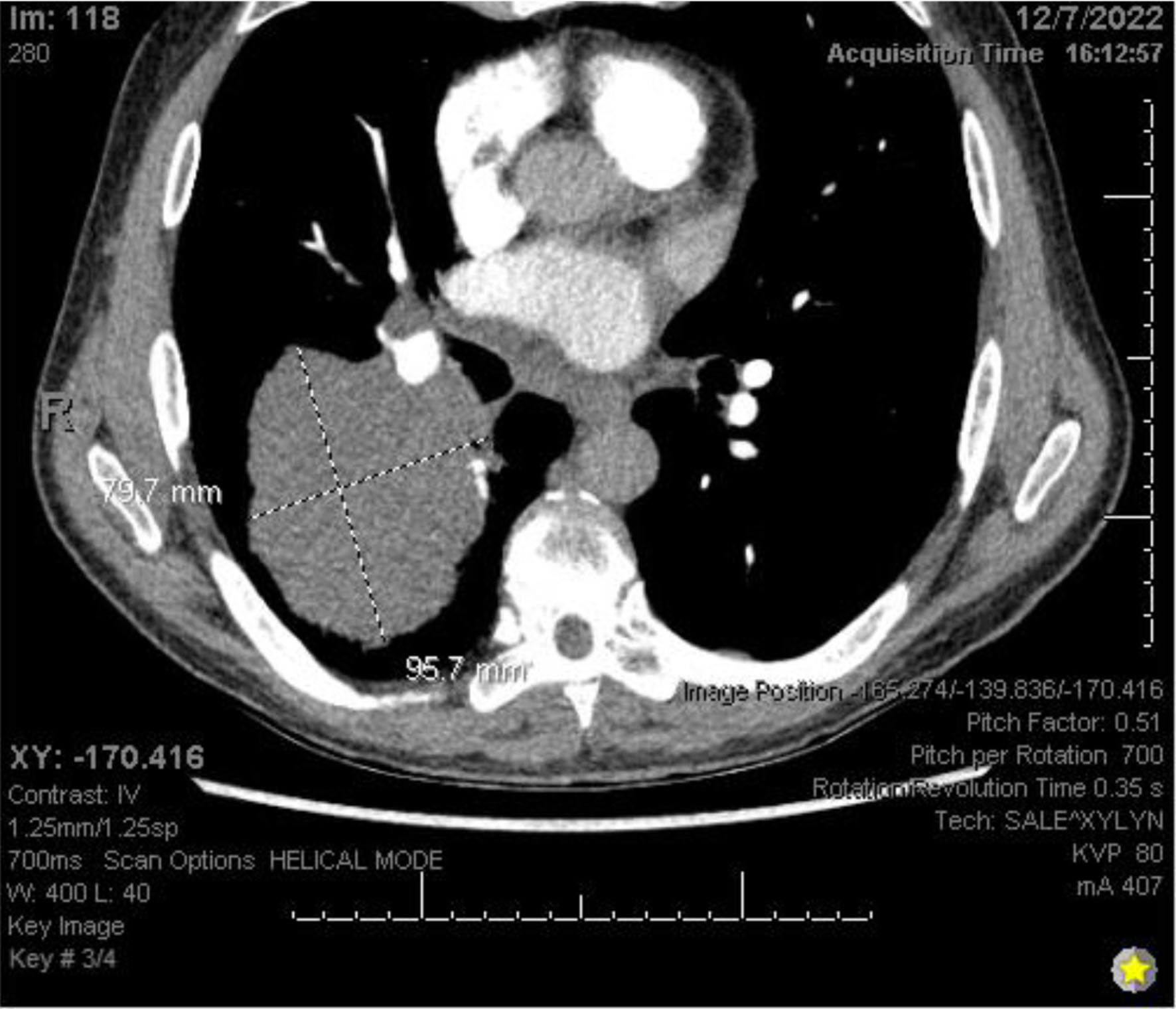 Click for large image | Figure 4. CTPA with IV contrast showing an approximately 9.6 × 8.0 cm mass in right lung. CTPA: computed tomography pulmonary angiography. |
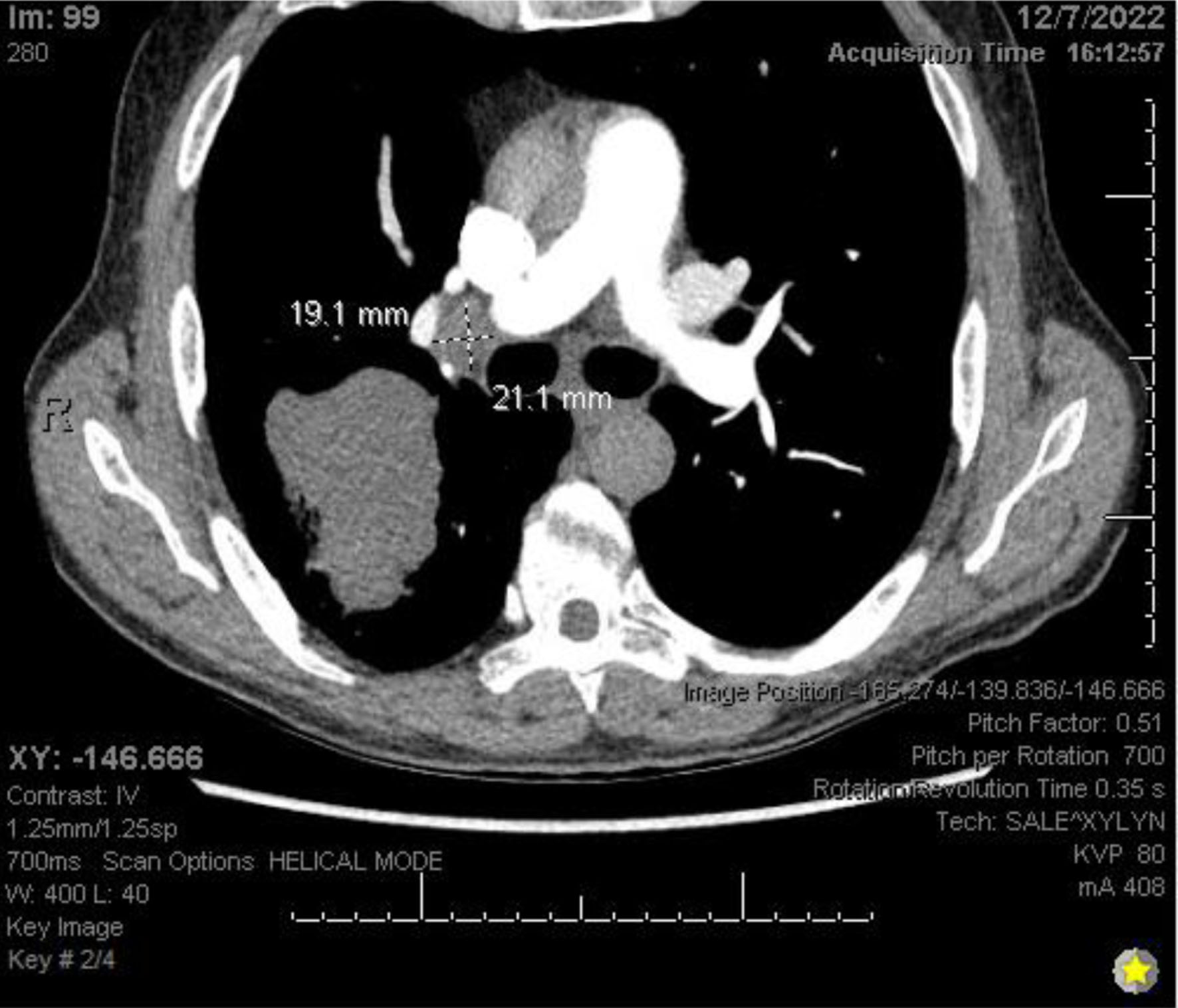 Click for large image | Figure 5. CTPA with IV contrast showing right hilar lymphadenopathy. CTPA: computed tomography pulmonary angiography. |
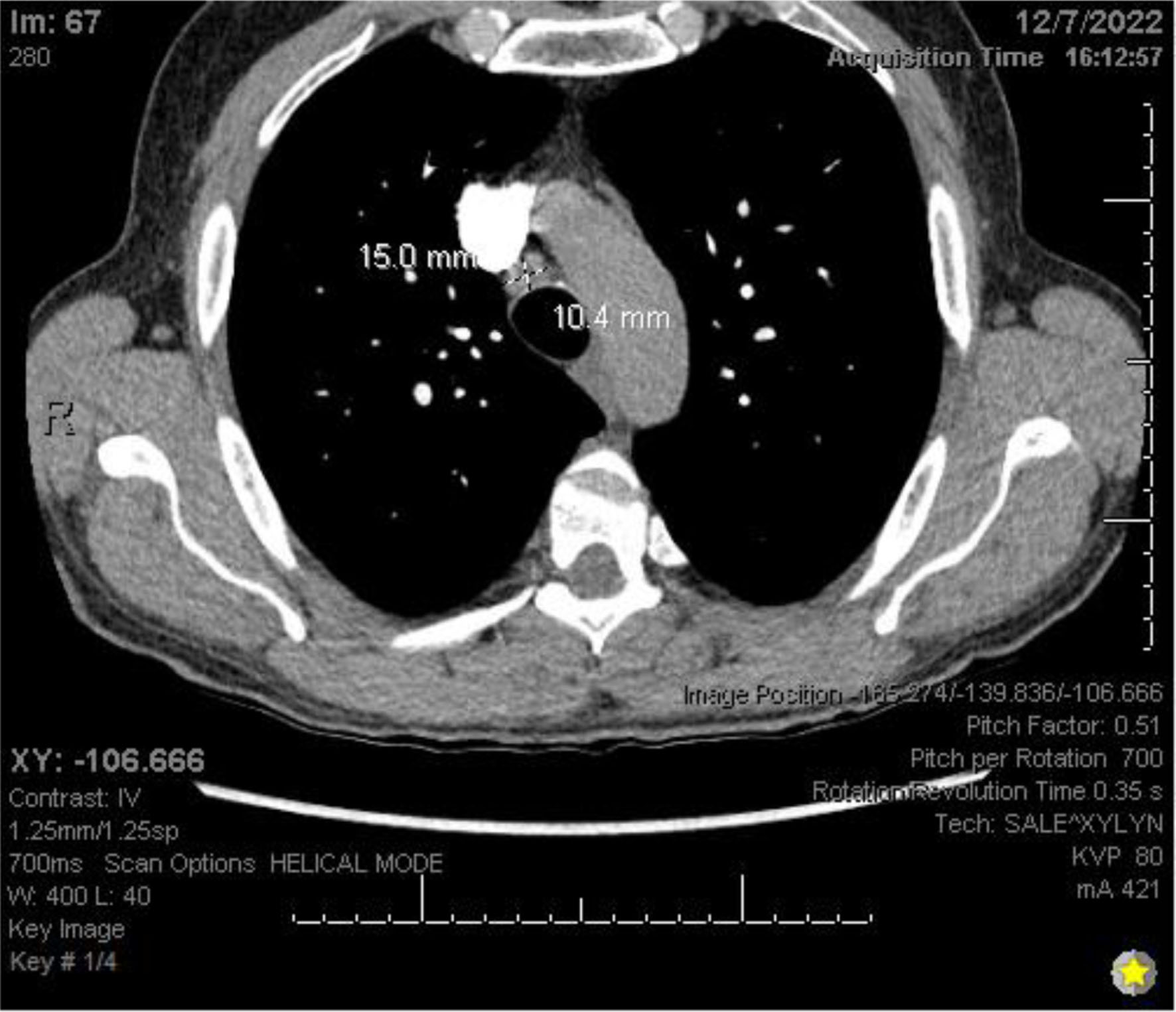 Click for large image | Figure 6. CTPA with IV contrast showing mediastinal lymphadenopathy. CTPA: computed tomography pulmonary angiography. |
Given the patient’s new-onset AF and newly diagnosed large lung mass highly concerning for malignancy, the patient received the appropriate workup for his new cardiopulmonary findings. Transesophageal echocardiogram (TEE) revealed a left ventricular ejection fraction (LVEF) of 60%, mild left atrial enlargement, and a large mass protruding from the right lower pulmonary vein into the left atrial cavity that fully obstructs flow in the corresponding pulmonary vein (Fig. 7).
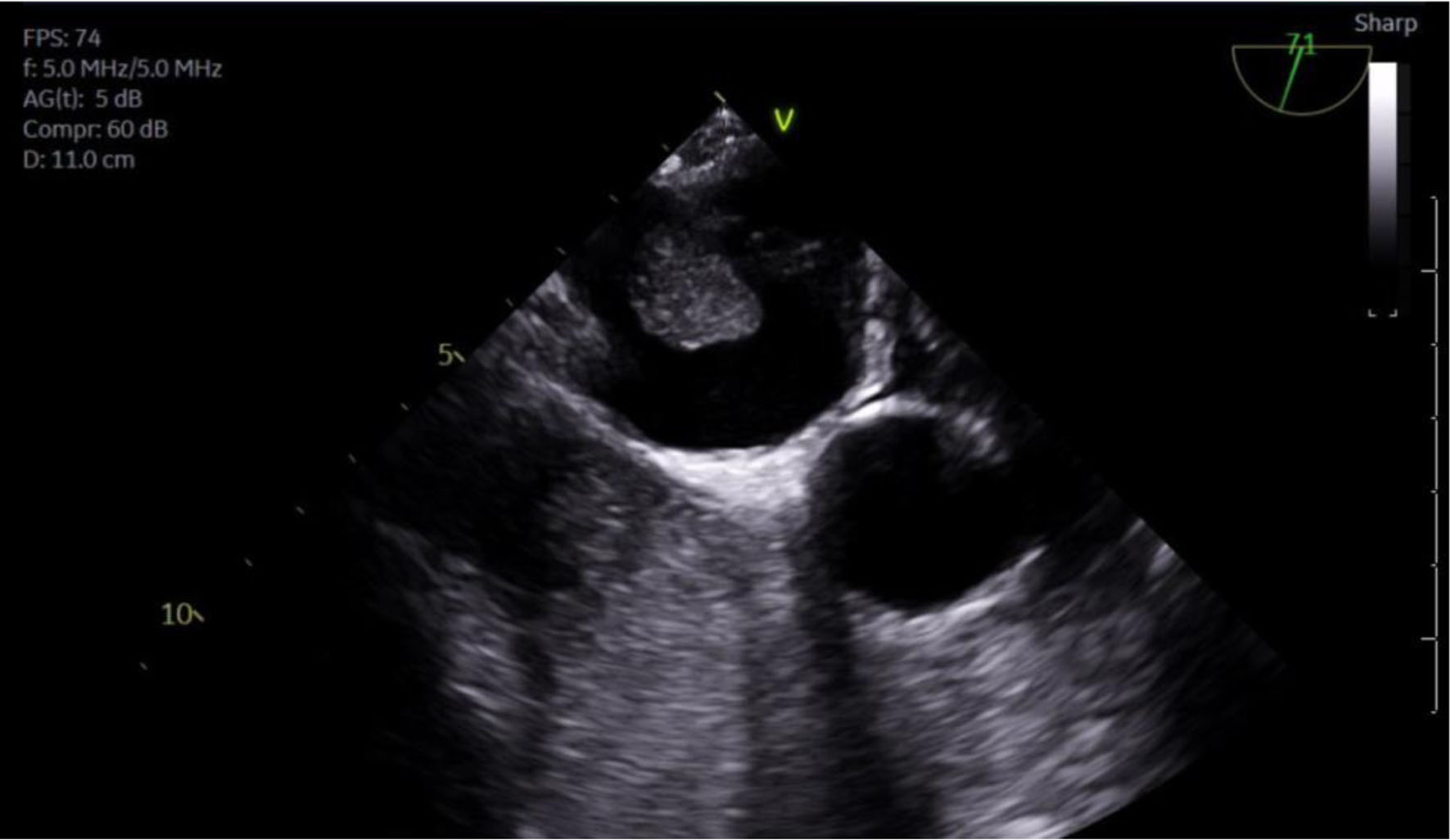 Click for large image | Figure 7. TEE showing mass protruding from PV into LA. PV: pulmonary vein; LA: left atrium; RV: right ventricle; TEE: transesophageal echocardiogram. |
Hematology/Oncology team was also consulted during the patient’s hospitalization and ordered positron emission tomography (PET)-CT of the whole body including the brain and abdomen/pelvis which showed no signs of metastatic disease. The patient underwent IR CT-guided lung biopsy of the large mass. After about 1 week, the pathology report described the mass as a poorly differentiated carcinoma with neuroendocrine features consistent with large cell high-grade neuroendocrine tumor of the lung.
Two days into hospitalization, the patient developed atrial flutter, confirmed on telemetry, which reverted back to sinus rhythm spontaneously. He was then started on metoprolol 25 mg twice daily for rate control and to prevent further arrhythmias. On day 4 of hospitalization, the patient went into AF with rate in the 120s-140s beats/min. He was given a single dose of diltiazem 10 mg and subsequently converted back into normal sinus rhythm. Later in the evening of day 4 of his hospital stay, the patient once again developed AF and was given three doses of diltiazem 10 mg which subsequent converted back to sinus rhythm. Given the multiple episodes of AF within a short period of time and many doses of diltiazem given, the patient’s metoprolol was increased to 50 mg twice daily. Despite this increase in his beta-blocker medication, the patient experienced an episode of AF on day 5 of hospitalization which was resistant to 5 mg of diltiazem and 5 mg of metoprolol given at the onset of the arrhythmia. His metoprolol was then further increased to 100 mg twice daily which converted him back into normal sinus rhythm after one dose. The patient in total was hospitalized for 7 days. He did not experience any episodes of AF during his finals 2 days, after his metoprolol was increased to 100 mg twice daily. Despite diltiazem converting the AF a number of times the patient was put on metoprolol because he was found to have bilateral essential hand tremor which was resolved when he started taking metoprolol while inpatient. The patient never experienced lightheadedness, headache, chest pain, palpitations, shortness of breath, or other associated symptoms during any of his AF episodes while in the hospital.
The patient was stable for discharge on the seventh day of his hospital stay and was medically discharged. The patient was given follow-up for outpatient oncology clinic. The patient was discharged on apixaban (Eliquis) 5 mg twice daily for primary anticoagulation, metoprolol 100 mg twice daily for rate control, lisinopril 20 mg daily for primary hypertension, and 3-day course of Percocet for moderate to severe pain.
| Discussion | ▴Top |
Few cases to date detail AF secondary to large lung malignancy. In our case, direct invasion of the left atria from the lung mass is the most likely underlying contributor to our patient’s condition. However, other factors are important to consider when determining the etiology of this patient’s presentation. A study by Bandyopadhyay et al examined the demographics of patients that have lung cancer and AF. Out of 159,615 patients with lung cancer, 10,050 (6.29%) of them had concurrent AF. In the patients with the diagnosis of AF and lung cancer, HTN, diabetes, dyslipidemia, and obesity were the most common comorbidities. Lastly, 46.7% of the 10,050 patients diagnosed with AF endorsed a past medical history significant for smoking [5]. Our patient’s extensive smoking history could have contributed to the patient’s condition since it is well known that smoking is an independent risk factor contributing to the incidence of AF [6, 7]. In addition, our patient experienced a 10-pound weight loss which is most consistent with a byproduct of a systemic pro-inflammatory state. A study by Yao et al details the role of the NLRP3 inflammasome signaling in cardiomyocytes. In patients with AF, NLRP3 inflammasome activity was increased in atrial cardiomyocytes [8]. To determine if NLRP3 activity contributed to the development of AF, the study utilized knock-in mice expressing active NLRP3 inflammasomes. The knock-in mice expressed an increase in ectopic electrical activity in their myocytes. Since our patient’s weight loss and shortness of breath began at a similar time, it is possible that the increased level of systemic inflammation enabled his myocytes to become more susceptible to ectopic beats. This additional factor is important to consider after evaluating our patient post-treatment since inflammatory activity may persist in the cardiac myocytes.
Several other cases document invasion of the left atrium either via the esophagus or via lung mass leading to AF in patients. One case describes an 80-year-old woman who had a 6.2 × 7.7 × 8.9 cm infracrainal mass that was penetrating the left atrium. This patient received four cycles of carboplatin and etoposide to decrease the size of her lung mass, and as a result, her AF symptoms decreased [9]. Another case illustrated a 69-year-old male who presented with syncope, shortness of breath on exertion, and a 37-pack-year smoking found to have refractory AF and rapid ventricular response secondary to a small cell carcinoma encapsulating the right pulmonary artery. In this case, the patient was given IV diltiazem and metoprolol pushes to control his AF; however, he reverted to RVRs between 130 and 150 beats/min. After the patient received his first round of chemotherapy, his heart rate decreased and converted to normal sinus rhythm with maintenance sotalol therapy [10]. While there are few cases like ours, one case report of a 58-year-old male who presented to the ER with dysphagia worsening with solids and a 10-kg unintentional weight loss over the span of 2 months matched closely with the presentation of our patient. This patient also endorsed a medical history of HTN on valsartan and hydrochlorothiazide and extensive smoking history. The patient was found to be in AF after initial presentation and further workup showed a 10-cm ulcerated mid-esophageal mass consistent with squamous cell carcinoma penetrating the left atrium [11]. The patient was controlled on a single dose of 20 mg of diltiazem and 25 mg of metoprolol twice daily.
Conclusion
The heart is an extremely sensitive organ functioning within a populated hemostatic space in the chest cavity. Mass effect and systemic responses from malignancy tend to be a recurring event in the presentation of patients with new-onset AF in the setting of cancer, especially of thoracic origin. The treatment modality chosen for our patient was a chemotherapy regimen, cisplatin and etoposide, on 21-day cycles with radiation therapy. Current literature suggests that patients with advanced large cell carcinoma of the lung have better prognoses when treated with chemotherapy and radiation [12]. One study demonstrated that patients with locally advanced stage III large cell neuroendocrine carcinoma treated with chemotherapy survived on average 11.9 months versus 16.1 months for patients receiving dual therapy with chemoradiation [13].
While not all cases of malignancy-related AF are alike, it is evident that the risk of serious cardiac arrhythmias increases drastically when external invasion of the heart is involved. It is imperative that individuals who are found to have malignancy, especially metastatic, have an appropriate cardiac workup and are watched on telemetry for development of AF and other cardiac conditions that may spontaneously occur. Future case reports and studies would be wise to focus on determining any relationship between specific location of malignancy and stratified risks for cardiac conditions if it exists. This will greatly benefit patient outcomes and lead to quicker and more efficient workups of malignant risk factors like the ones discussed in our case report.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent from the patient to publish this case was obtained.
Author Contributions
Ali Rahman contributed to study design, manuscript development and editing. Sura Alqaisi contributed to manuscript development and editing. Shiv Krishnaswamy and Emilio Hospedales contributed to manuscript writing, data collection, and figures arrangement. Walif Aji contributed to oversight and editing of final manuscript.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article.
| References | ▴Top |
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946-952.
doi pubmed - Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
doi pubmed - Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840-844.
doi pubmed - van der Hooft CS, Heeringa J, van Herpen G, Kors JA, Kingma JH, Stricker BH. Drug-induced atrial fibrillation. J Am Coll Cardiol. 2004;44(11):2117-2124.
doi pubmed - Bandyopadhyay D, Ball S, Hajra A, Chakraborty S, Dey AK, Ghosh RK, Palazzo AM. Impact of atrial fibrillation in patients with lung cancer: Insights from National Inpatient Sample. Int J Cardiol Heart Vasc. 2019;22:216-217.
doi pubmed - Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156(6):1163-1169.
doi pubmed - Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ, Loehr LR, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129(9):971-980.
doi pubmed - Yao C, Veleva T, Scott L, Jr., Cao S, Li L, Chen G, Jeyabal P, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138(20):2227-2242.
doi pubmed - Ahmed N, Carlos MM, Moshe G, Yitzhak R. Association between left atrial compression and atrial fibrillation: a case presentation and a short review of literature. J Atr Fibrillation. 2016;9(2):1458.
- Emmanuel KE, Jensen N, Anyanwagu U. Refractory Atrial Fibrillation With Rapid Ventricular Rate in a Patient With Small Cell Carcinoma of the Lung Encasing the Right Pulmonary Artery: A Case Report and Insight Into Therapeutic Options. Cureus. 2021;13(6):e16027.
doi - Bayraktar UD, Dufresne A, Bayraktar S, Purcell RR, Ajah OI. Esophageal cancer presenting with atrial fibrillation: a case report. J Med Case Rep. 2008;2:292.
doi pubmed - Gu J, Gong D, Wang Y, Chi B, Zhang J, Hu S, Min L. The demographic and treatment options for patients with large cell neuroendocrine carcinoma of the lung. Cancer Med. 2019;8(6):2979-2993.
doi pubmed - Limonnik V, Abel S, Finley GG, Long GS, Wegner RE. Factors associated with treatment receipt and overall survival for patients with locally advanced large cell neuroendocrine carcinoma of the lung: A National Cancer Database analysis. Lung Cancer. 2020;150:107-113.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


