| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 6, December 2022, pages 372-379
Perioperative Validation of the TensorTip™ MTX Device for Noninvasive Arterial Pressure Measurement: A Method Comparison Study
Sjoerd Servaasa, b , Lucas T. van Eijka
, Silke de Vreedea
, Ignacio Malagona
, Cornelis Slagta
aDepartment of Anaesthesiology, Pain and Palliative Medicine, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands
bCorresponding Author: Sjoerd Servaas, Department of Anaesthesiology, Pain and Palliative Medicine, Radboud University Medical Centre, 6500 HB Nijmegen, The Netherlands
Manuscript submitted September 12, 2022, accepted October 22, 2022, published online December 16, 2022
Short title: BP Measurements by TensorTip MTX and AC
doi: https://doi.org/10.14740/cr1438
| Abstract | ▴Top |
Background: The noninvasive TensorTip™ MTX measures blood pressure by interpreting blood diffusion color of the finger skin. In addition to blood pressure, the device is able to measure various vital signs: heart rate, oxygen saturation, stroke volume, and cardiac output. Studies about accuracy and precision thus far available have only been conducted by the manufacturer. The aim of our study was to investigate the accuracy and precision of the TensorTip MTX in comparison to invasive radial artery blood pressure values.
Methods: Forty-one patients scheduled for elective surgery were enrolled in this study. Placement of the arterial catheter had to be part of the standard of care. Once hemodynamic stable conditions were achieved, blood pressure was measured. Three measurements with the TensorTip MTX were averaged and compared with one invasive blood pressure measurement using Bland-Altman plot and error grid analysis.
Results: Systolic, diastolic, and mean blood pressure had a bias of respectively 6.2, -6.9 and 4.4 mm Hg. Corresponding standard deviation were respectively 30.1, 17.0 and 22.2. Calculated percentage errors were 47.6%, 52.9% and 52.3%. Proportional bias was present in all Bland-Altman analyses. Error grid analysis showed 61.0% of systolic blood pressure measurements, and 46.3% of mean blood pressure measurements were in the clinical acceptable zone.
Conclusions: The TensorTip MTX was not able to reliably measure blood pressure compared to blood pressure obtained with an arterial catheter and therefore, the measurement performance is not clinically acceptable. Moreover, a high malfunction rate makes the device unsuitable for use in perioperative period.
Keywords: Blood pressure; Comparison study; TensorTip MTX; Measurement equipment
| Introduction | ▴Top |
Continuous blood pressure (BP) monitoring is desirable in hemodynamic unstable patients and during procedures where hemodynamic instability is likely to occur. It may be desirable to continuously monitor BP on the ward after the acute phase (e.g., emergency room, operating room or intensive care unit) enabling a quick response to hemodynamic changes [1]. A prompt reaction to hemodynamic changes may reduce the total time of hypotension and theoretically lead to a reduction in hemodynamic adverse events [2]. Measurement of continuous BP is ideally performed with an arterial catheter [3]. Intra-arterial catheter placement is associated with complications such as thrombosis, infection, and ischemia [4]. These serious adverse effects of arterial catheter placement are witnessed sporadically (< 1%), however temporary radial artery occlusion and hematomas are reported more often [4]. The use of a noninvasive method would prevent the risk of developing such complications. Where an arterial catheter is not used in the general ward, oscillometry or a sphygmomanometer is used. Both methods have a margin of error relative to the artery catheter and because measurements are performed at a lower frequency, there may be a delay in the diagnosis and treatment of hemodynamic adverse events [2, 5]. Various techniques for noninvasive BP measurements have been developed [6]. However, these methods are not widely implemented, mainly due to lack of proper precision and accuracy compared to BP obtained with an arterial catheter.
The TensorTip MTX (MTX, Cnoga Medical Ltd., Caesarea, Israel) is a device that measures BP noninvasively (Fig. 1). The device is also able to measure heart rate, oxygen saturation and cardiac output. In addition, the device can also measure blood values, such as hemoglobin and blood gas analysis (outside the focus of this study). The device encircles the finger of the patient and seals off all environmental sources of light. Four monochromatic light sources (about 600 nm to about 1,000 nm) radiate light with different wavelengths through the finger’s capillary tissue. This light is then projected on a color image sensor, used as a three-dimensional (3D) spectrometer and color distributor. With different observed colors (red, blue, green), measured in two positions and over time, the blood diffusion color of the skin is analyzed. Use of the algorithms designed by the manufacturer - the color information relative to place and time - allows for the calculation of vital parameters [7].
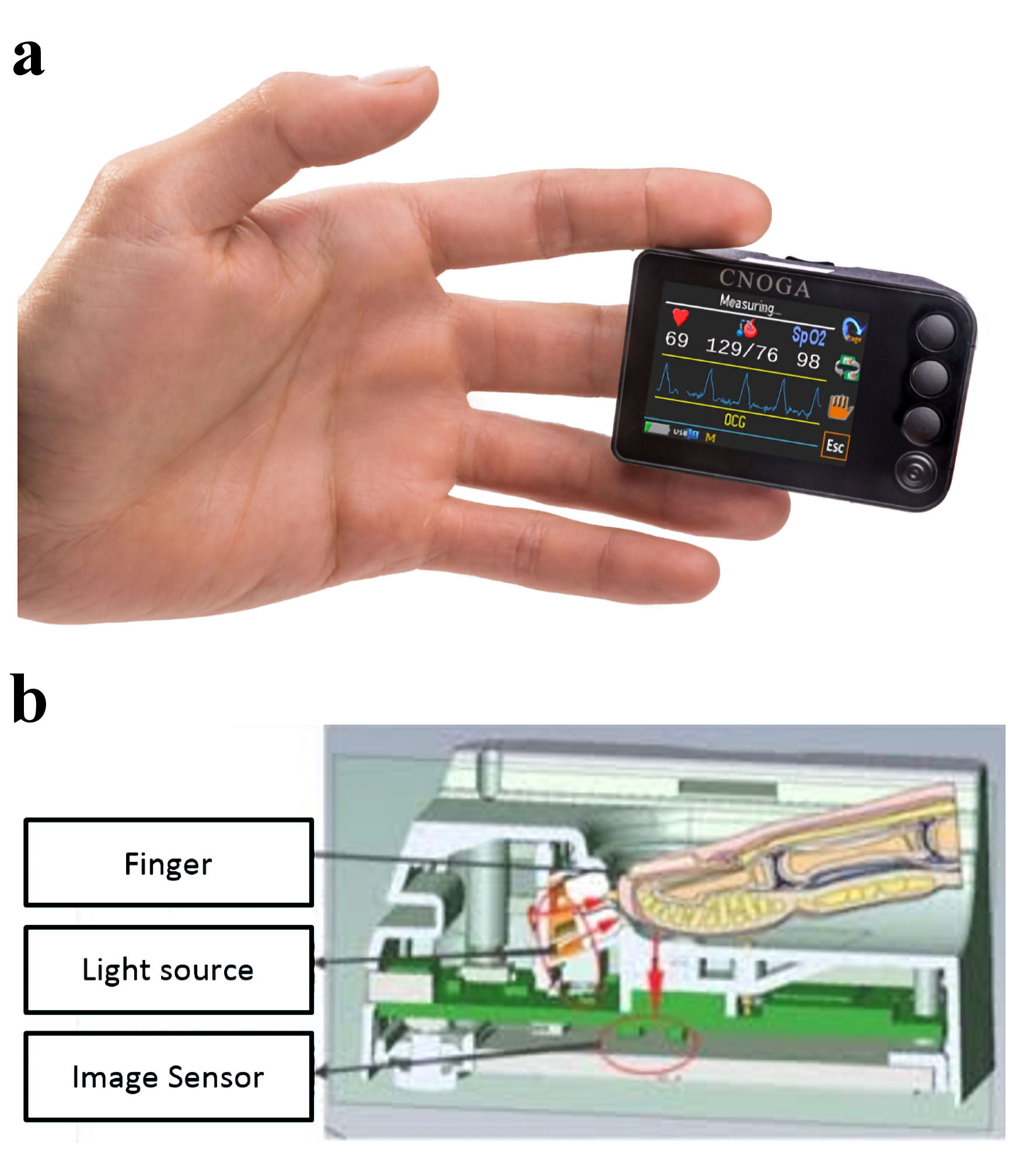 Click for large image | Figure 1. The TensorTip MTX. (a) View of the TensorTip MTX with correct placement. (b) The lower part of the figure shows how measurements technically are performed. |
The TensorTip MTX is a relative new device. To our knowledge, the studies thus far available have solely been conducted by the manufacturer [7-9]. The conditions that a BP device must meet, in order to be approved, are clearly stated in the universal standard (the Association for the Advancement of Medical Instrumentation (AAMI)/European Society of Hypertension (ESH)/International Organization for Standardization (ISO)) for the validation of BP measuring devices [5]. A comparison study should show a maximum bias of 5 mm Hg with maximal standard deviation (SD) of 8 mm Hg in order to approve a new device. It is also recommended to analyze the clinical relevance of the differences between two methods [10].
The aim of this study was to evaluate the precision and accuracy of BP measurements using the TensorTip MTX compared with BP measurements with an arterial catheter throughout the perioperative period.
| Materials and Methods | ▴Top |
Ethics
This study was conducted according to the Declaration of Helsinki and in accordance with the International Conference on Harmonization (ICH) guidelines for Good Clinical Practice. Ethical approval for this study (Ethical committee No. 2020-6660) was provided by the Medical Ethical Committee Arnhem-Nijmegen, the Netherlands (chairman Prof Dr P.N.R. Dekhuijzen) on June 18, 2020. This study was registered at www.trialregister.nl with national trail registry number NL9164. Data were obtained between November 2020 and August 2021.
Study design and subjects
This single-center, prospective observational study was conducted by the Department of Anesthesia, Pain and Palliative Medicine at the Radboud University Medical Center, Nijmegen, The Netherlands. Eligible to take part in this study were patients who were ≥ 18 years old and who required continuous invasive arterial BP monitoring as part of the standard clinical care. Patients with anatomical abnormalities preventing application of the TensorTip MTX and patients suspected of or with proven coronavirus disease 2019 (COVID-19) infection were excluded from this study. Participation in this study did not interfere with standard perioperative care. All patients gave written informed consent.
Hemodynamic monitoring and measurements
Measurements were performed at three pre-defined time points. Shortly after induction, before emergence of anesthesia and after surgery at the post anesthetic care unit. Patients were in the supine position, with the finger at the height of the heart. After induction of anesthesia, an arterial catheter was placed in the radial artery (Becton Dickinson arterial cannula 20G/1.10 mm × 45 mm, Becton Dickinson infusion therapy Systems Inc. Utah, USA) and calibrated accordingly. Invasive arterial BP measurements were directly visible and stored for analysis (IntelliVue MX800 or IntelliVue MP70 monitor, Philips, The Netherlands, software version J.10.52).
The TensorTip MTX device was set up according to the operational manual provided by the manufacturer. This means that before using the device itself, a self-test was performed. Subsequently, a “virtual arm cuff” had to be selected. Options were large (male with cold fingers, male/female with high body mass index (BMI), or male/female suspected for high BP), medium (male with normal BMI or female with cold fingers), small (small BMI) or automatically (device will adjust automatically). To keep conditions the same for all patients, we selected the automatic adjustment option [11]. The device was applied to the middle finger at the same side as the arterial catheter. If the middle finger did not show results, the index finger or ring finger was used. Three BP measurements were taken with the TensorTip within a 1-min time frame. At the time of the second measurement the invasive BP value of the arterial catheter was recorded. As all measurements were performed under hemodynamic stable conditions, we chose to record only one measurement from the invasive arterial catheter. The three measurements of the TensorTip MTX were averaged. The mean value was compared with the single value of the arterial catheter. The measurements were recorded and saved in an application named “Singular” (CnogaCare, Cnoga Medical Ltd., Caesarea, Israel). This application is freely available and designed to connect with the TensorTip MTX.
Statistical analysis
Statistical analyses were performed using IBM SPSS statistics 25 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5.03 (GraphPad software, Inc, La Jolla, CA). Data are available on request.
There are no known margins of error for the TensorTip MTX, so a calculation of sample size is not possible. We used the principle, described by Viechtbauer et al to calculate the sample size [12]. This principle provides a method for sample size calculation for pilot studies based on the problem probability. We anticipated a problem probability of 6% with a confidence of 95%. With this percentage we calculated a group of N = Ln(1 - 0.95)/Ln(1 - 0.06) = 49 patients, rounded up to 50. Patient characteristics and hemodynamic parameters were assessed using descriptive analysis and reported as mean ± SD. Data were checked for normal distribution using the Shapiro-Wilk test and by visual interpretations of the quantile-quantile plot. Systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean blood pressure (MBP) were assessed separately. The precision of the tested method was calculated using the three consecutive measurements. Bland-Altman analysis was used for calculation of bias, limits of agreement (LoA) and percentage error (PE), all accompanied with a confidence interval (CI) of 95% [13]. The PE was calculated as follows:
Regression analysis was performed to assess proportional bias. A maximum bias of 5 mm Hg with maximal SD of 8 mm Hg (applicable for SBP, DBP, and MBP) should be observed in order to show good precision and accuracy [5]. Only when a tested device complies with these criteria it can replace the reference device. For the evaluation of clinical relevance, we used the error grid method suggested by Saugel et al for SBP and MBP [10].
The error grid method is designed to illustrate the clinical relevance of measured differences of two methods. Based on survey amongst 25 specialists in anesthesia and intensive care medicine, the error grid was constructed with five risk zones. Zone A depicting measurements with “no risk” up to zone E showing “dangerous risk”. The idea of this method is that measurements in the no risk group can differ statistically from each other, but the difference is clinically not important. The clinical difference between two methods was defined as acceptable if at least 90% of the measurements were located in zone A.
| Results | ▴Top |
Fifty-three patients were included in the study. Patient characteristics are presented in Table 1. Twelve patients were excluded from final statistical analysis because the TensorTip MTX malfunctioned and led to loss of data. The device was repeatedly unable to display hemodynamic values despite passing the self-test. Data from 41 patients was available for statistical analysis. Correlation plots for SBP, DBP, and MBP are presented in Figure 2, together with their respective correlation coefficients (r). Table 2 shows the mean measured SBP, DBP, and MBP of measurements obtained with the radial artery catheter and the TensorTip MTX. Shapiro-Wilk test showed normal distribution for SBP, DBP and MBP.
 Click to view | Table 1. Patient Characteristics |
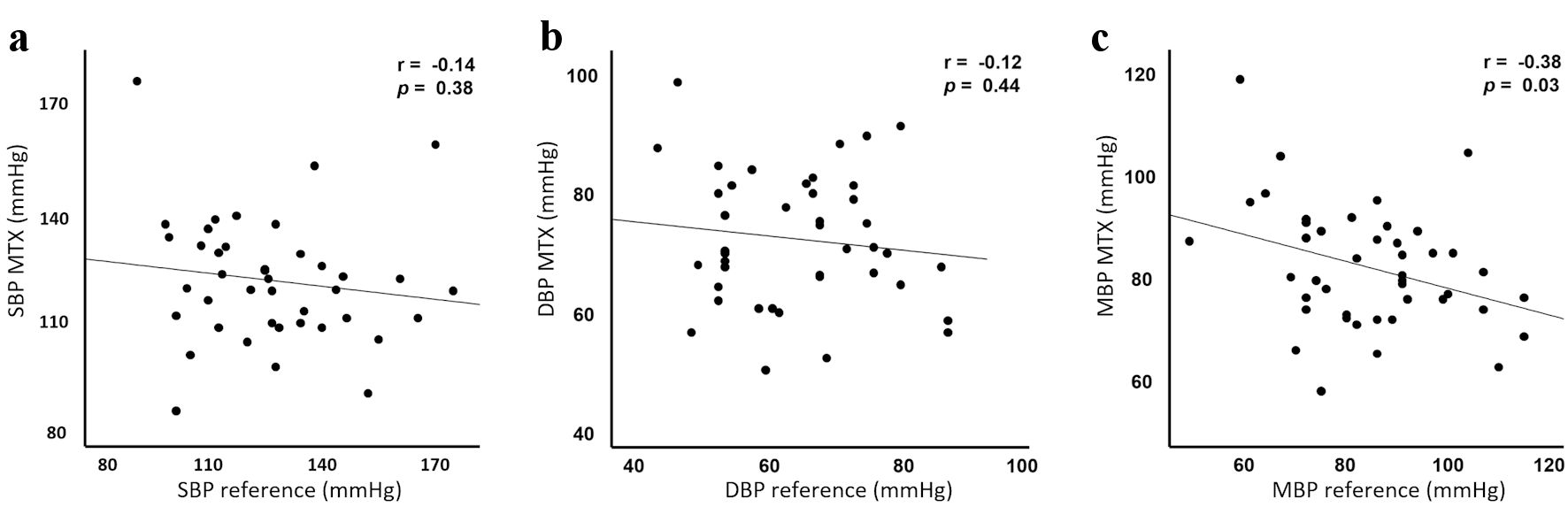 Click for large image | Figure 2. Correlation analysis. Correlation analysis for systolic (a), diastolic (b) and mean (c) blood pressure comparing TensorTip MTX with arterial catheter. SBP and DBP show negligible correlation. MBP shows low negative correlation. The correlation coefficient (r) is shown in the top corner of the three figures. SBP: systolic blood pressure; DBP: diastolic blood pressure; MBP: mean blood pressure. |
 Click to view | Table 2. Mean Blood Pressure |
SBP
The range of invasive SBP was 89.0 to 178.0 mm Hg with a mean of 127.1 ± 22.0 mm Hg. SBP obtained with the TensorTip MTX had a range of 84.3 to 176.7 mm Hg with a mean of 120.9 ± 17.7 mm Hg. Measurements of the TensorTip MTX had a precision of 6.2% for SBP. Figure 3 shows the Bland-Altman analysis. Bias was 6.2 (95% CI: -3.0 to 15.4, SD: 30.1) mm Hg. LoA’s were -52.8 (95% CI: -68.8 to -36.8) and 65.2 (95% CI: 49.2 to 81.2) mm Hg. The calculated PE was 47.6% (95% CI: 34.7 to 60.4).
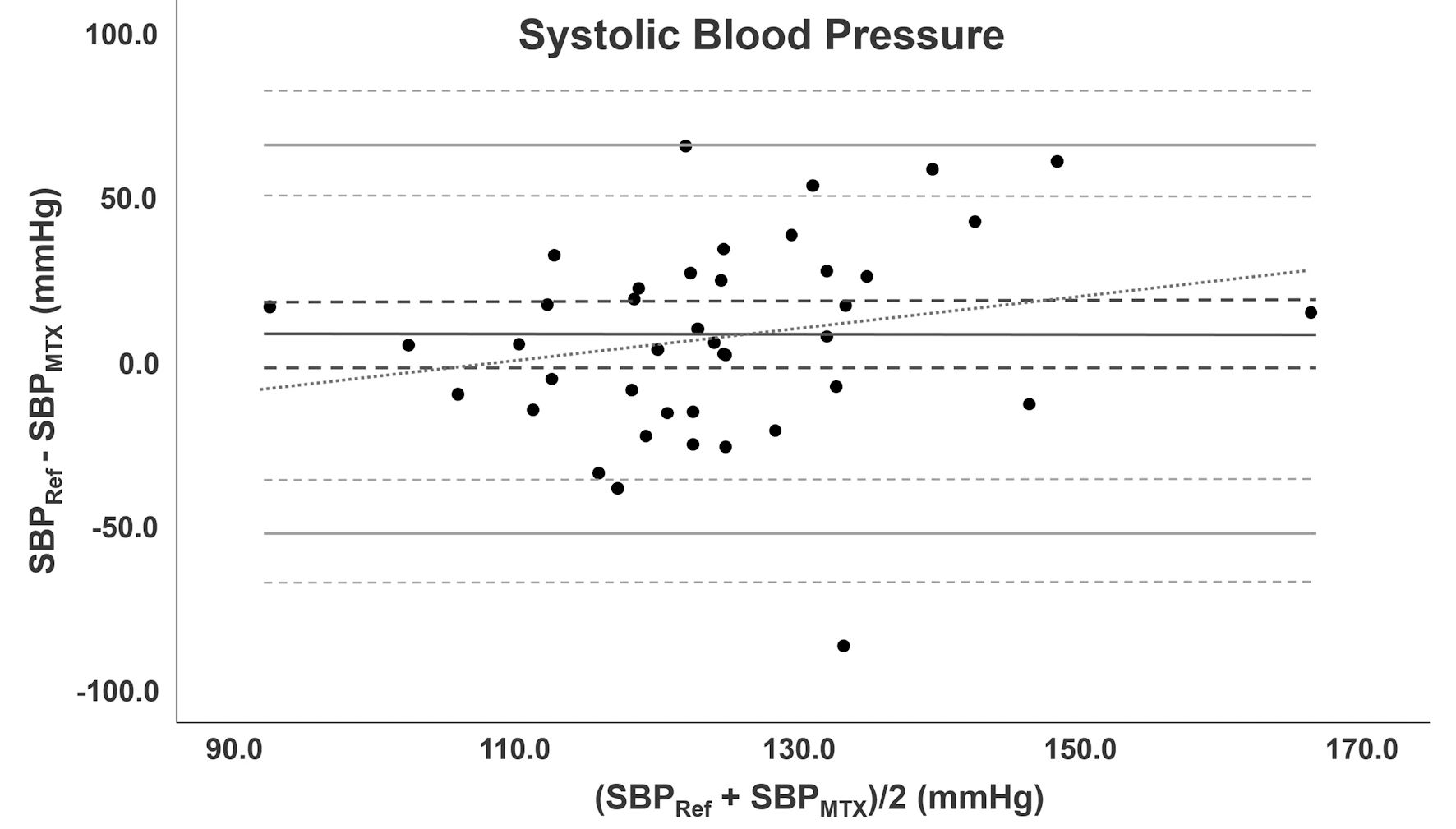 Click for large image | Figure 3. Bland-Altman analysis for SBP. Bland-Altman analysis for SBP comparing TensorTip MTX with AC. Bias is depicted as black line accompanied with confidence intervals (dotted lines). The grey lines represent LoA with confidence intervals. The diagonal line represents the proportional bias. SBP: systolic blood pressure; AC: arterial catheter; LoA: limit of agreement. |
DBP
Bland-Altman analysis for DBP is depicted in Figure 4. Invasive DBP measurements had a range of 44.0 to 87.0 mm Hg with a mean of 64.8 ± 11.5 mm Hg. The range of TensorTip MTX DBP was 49.7 to 98.0 mm Hg and mean 71.7 ± 11.2 mm Hg. Measurements of the TensorTip MTX had a precision of 8.0% for DBP. The bias was -6.9 (95% CI: -12.1 to -1.7, SD: 17.0) mm Hg. LoA’s were -40.3 (95% CI: -49.2 to -31.2) and 26.4 (95% CI: 17.3 to 35.4) mm Hg. PE was 48.8% (95% CI: 35.6 to 62.0).
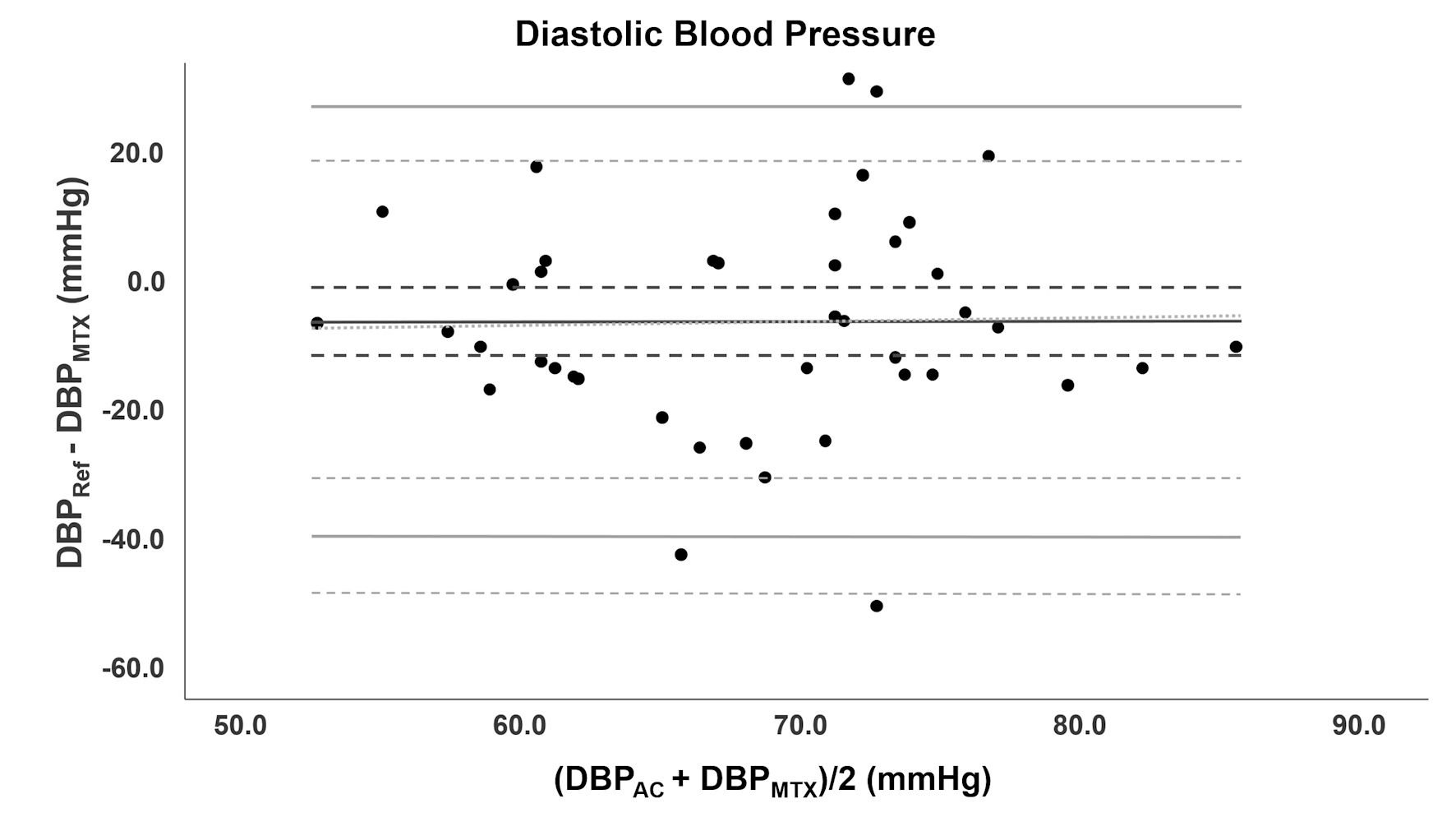 Click for large image | Figure 4. Bland-Altman analysis of DBP. Bland-Altman analysis for DBP comparing TensorTip MTX with AC. Bias is depicted as black line accompanied with confidence intervals (dotted lines). The grey lines represent LoA with confidence intervals. DBP: diastolic blood pressure; AC: arterial catheter; LoA: limit of agreement. |
MBP
Figure 5 shows the Bland-Altman analysis for MBP. The range of MBP was 50.0 to 116.0 mm Hg obtained with an arterial catheter and 57.0 to 118.0 mm Hg with the TensorTip MTX. Mean was 85.6 ± 15.3 mm Hg and 81.2 ± 11.8 mm Hg for BP obtained with arterial catheter and TensorTip MTX, respectively. Measurements of the TensorTip MTX had a precision of 8.1% for MBP. Bias was 4.4 mm Hg (95% CI: -2.4 to 11.2, SD: 22.2). LoA’s were -39.1 (95% CI: -50.9 to -27.3) and 47.9 (95% CI: 36.1 to 59.7) mm Hg. PE was 52.2% (95% CI: 38.1 to 66.3).
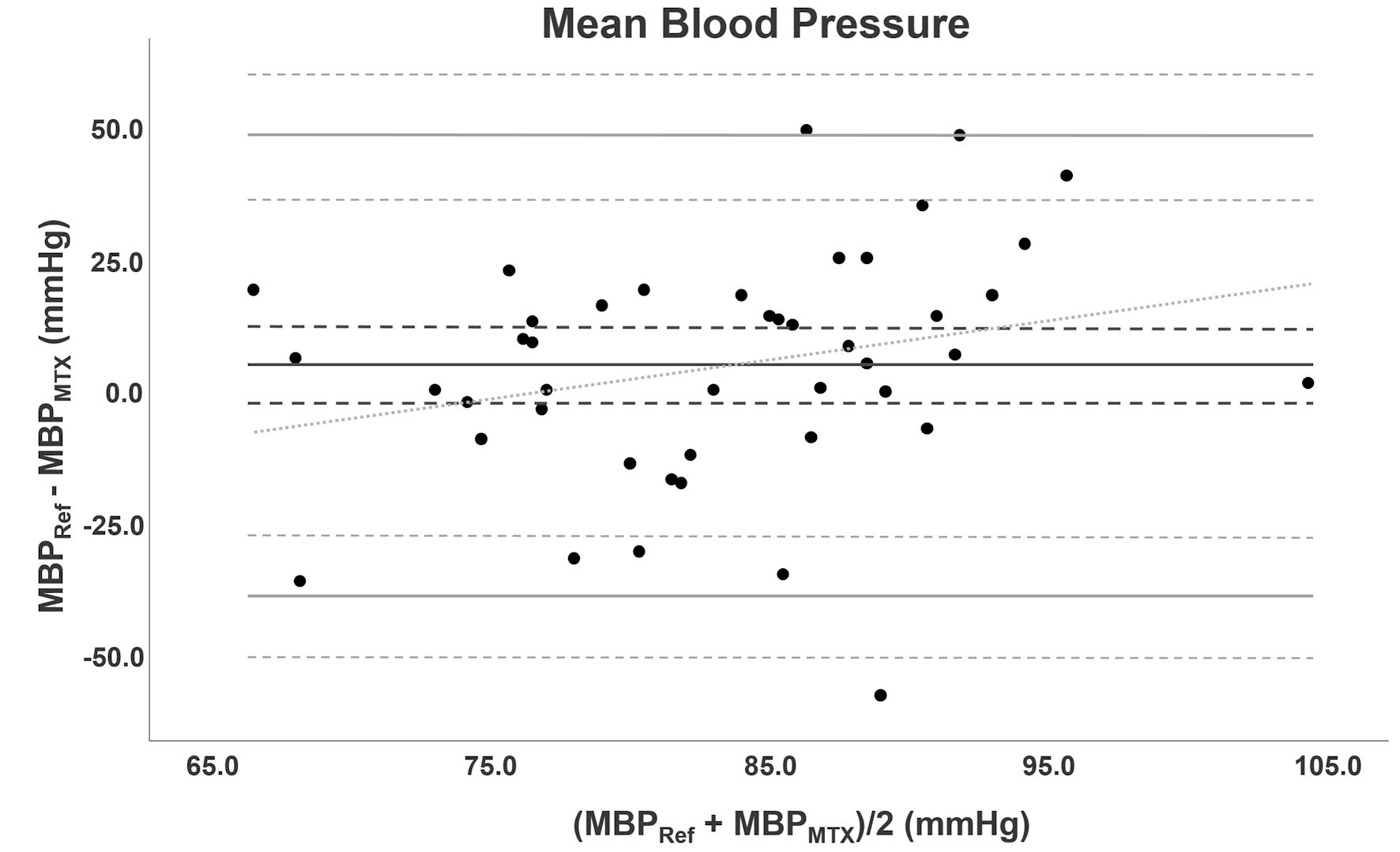 Click for large image | Figure 5. Bland-Altman analysis of MBP. Bland-Altman analysis for MBP comparing TensorTip MTX with AC. Bias is depicted as black line accompanied with confidence intervals (dotted lines). The grey lines represent LoA with confidence intervals. The diagonal line represents the proportional bias. MBP: mean blood pressure; AC: arterial catheter; LoA: limit of agreement. |
Error grid analysis
Error grid analysis were performed for SBP and MBP. Figure 6 shows the error grid analysis of SBP. From 41 measurements 25 points (61.0%) were located in zone A. Zone B included 12 points (29.2%) and zones C and D respectively 3 (7.3%) and 1 (2.4%) points. The error grid analysis of MBP is shown in Figure 7. Zone A included 19 points (46.3%), 14 points (34.1%) were located in zone B, 6 points (14.6%) in zone C and 2 points (4.9%) in zone D.
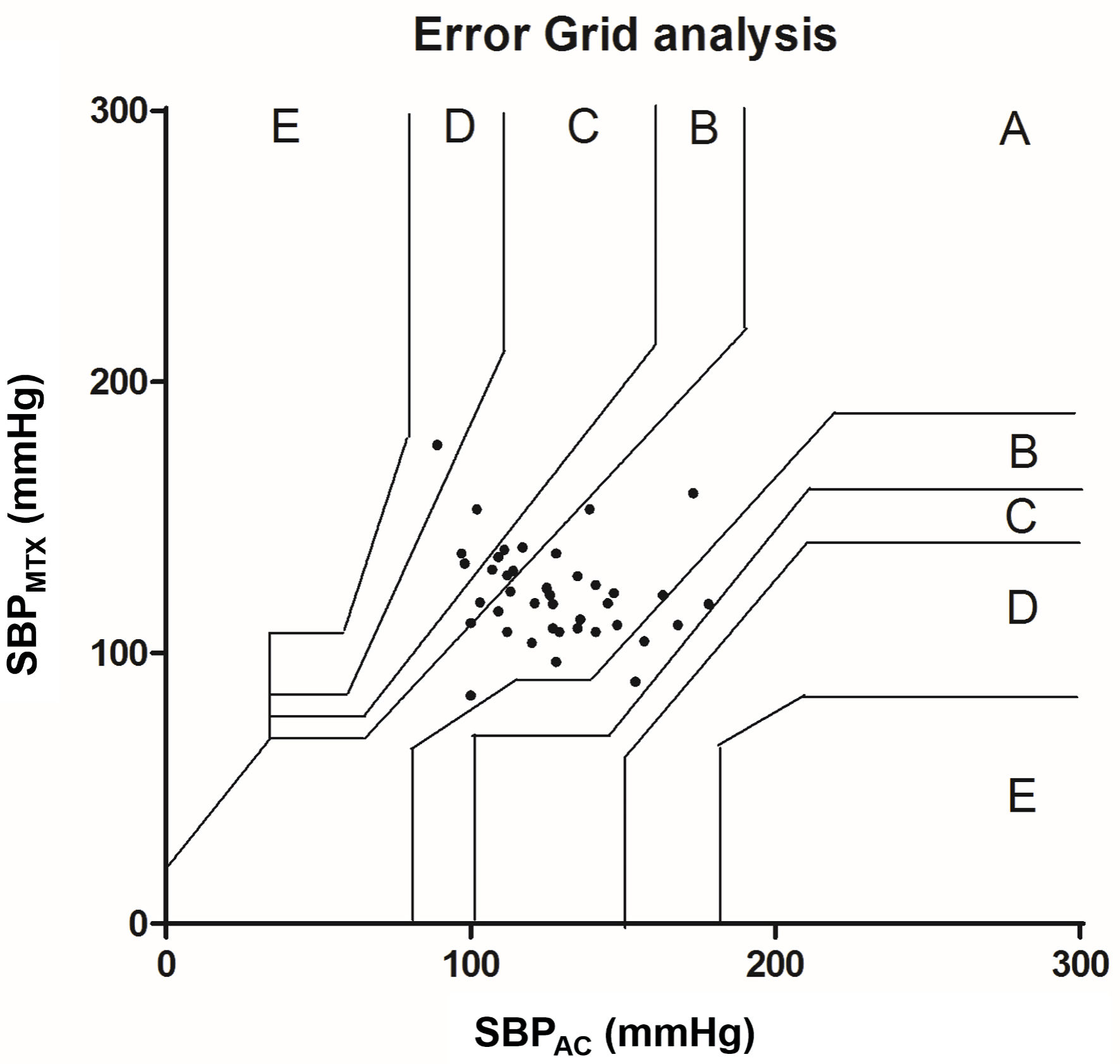 Click for large image | Figure 6. Error grid analysis for SBP. The error grid is divided in five zones: A - E: each zone represents a risk level for treatment error. A: no risk, B: low risk, C: moderate risk, D: significant risk and E: dangerous risk. SBP: systolic blood pressure; AC: arterial catheter. |
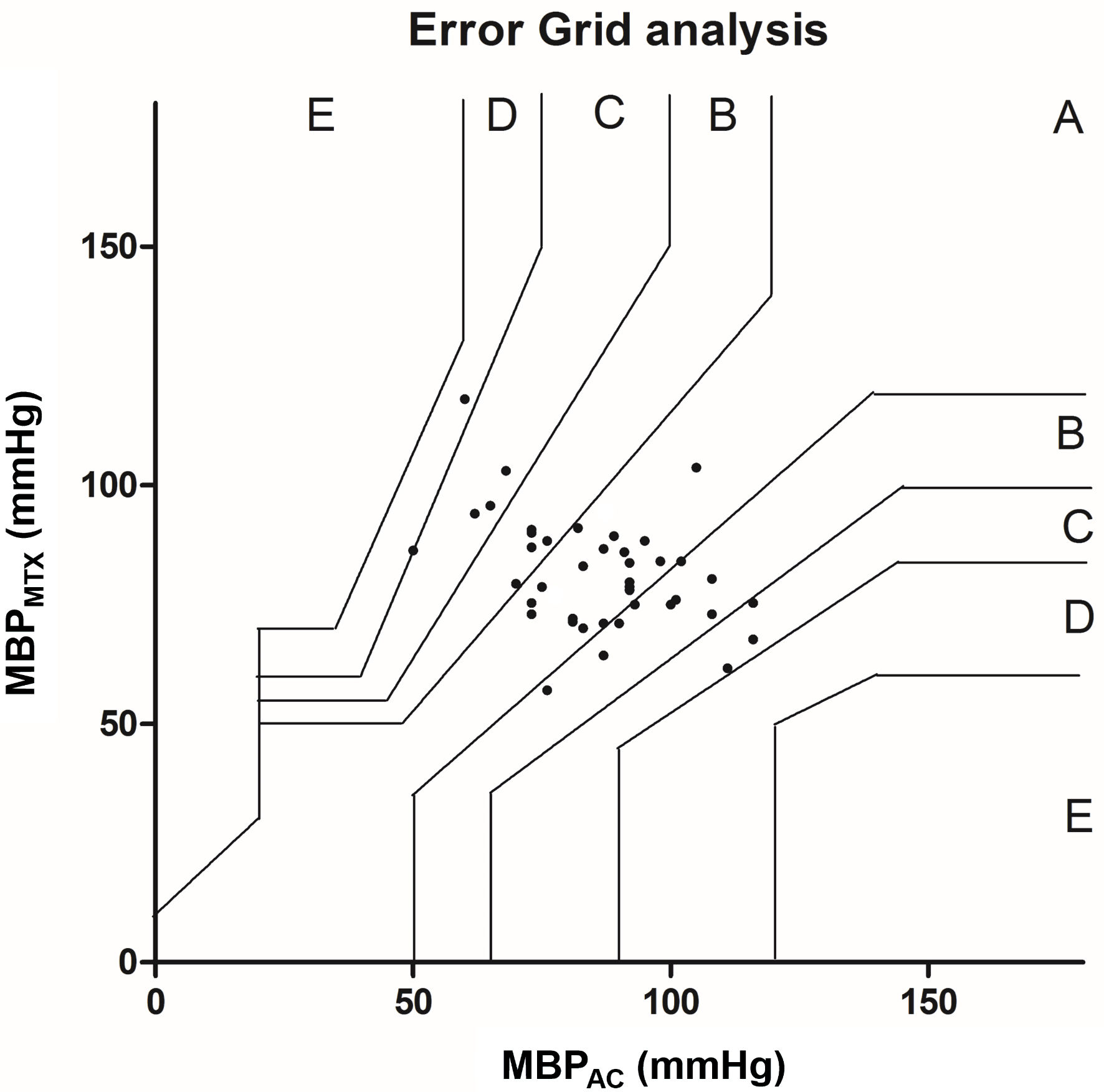 Click for large image | Figure 7. Error grid analysis for MBP. The error grid is divided in five zones: A - E: each zone represents a risk level for treatment error. A: no risk, B: low risk, C: moderate risk, D: significant risk and E: dangerous risk. MBP: mean blood pressure; AC: arterial catheter. |
| Discussion | ▴Top |
This study compares noninvasive BP measurements assessed by the TensorTip MTX to invasive BP obtained with an arterial catheter in the perioperative setting. Our study shows that BP measurements obtained with the noninvasive TensorTip MTX deviate substantially from measurements obtained with an invasive arterial catheter. The differences between the two methods are also clinically relevant.
The results from SBP, MBP, and DBP show a bias > 5 mm Hg with SD exceeding 8 mm Hg. As a result, the device does not meet the criteria required to replace the reference method with the assessed new monitor [5]. It should be noted here that the AAMI standard only mentions these limits for SBP and DBP. There is no reason to assume that this should be any different for MBP. In our analysis we found a mean PE of respectively 47.6%, 52.9%, and 52.3% for SBP, DBP, and MBP. PE is not regularly used when comparing BP devices. There is no consensus on the acceptable limits. While for CO measurements, this is set at 30% [14]. It is plausible that this percentage will be lower for BP devices, considering the aforementioned requirements of the bias and SD. With the bias, SD, LoA and PE found in our study, this device performs less well compared to radial artery applanation tonometry or finger arterial pressure [15, 16]; two other noninvasive continuous BP devices that have been studied in the past. Two validation studies from Segman et al evaluated the TensorTip MTX with BP obtained with arterial catheter in two different medical centers, under different clinical conditions [7, 8]. Both studies concluded that the TensorTip MTX proved to be accurate. However, no Bland-Altman analysis for BP measurements was presented and the correct assessment criteria (bias < 5 mm Hg with SD not exceeding 8 mm Hg) were missing. Our study also shows the presence of proportional bias, decreasing accuracy and precision as BP increases [17, 18].
The error grid analysis shows in both SBP and MBP a percentage in zone A below 90%, therefore clinically unacceptable. The use of 90% as a limit is subject to debate, nevertheless, is commonly used [10, 19]. It has been suggested to merge zones A and B as an acceptable option [20]. In our study this would mean that error grid analysis for SBP would become clinically acceptable, while MBP remains unacceptable.
Our results show a malfunction of the device in 12 of our 53 included patients (23%). In some patients this was probably due to cold digits. This problem has been described by Segman et al in a post cardiac surgery population [7]. However, their measurement problem was described in only 7.5% of the cases. While measuring, the device gave an error message if the contact between the finger and the sensor was not adequate, or when the hand was moved ever so slightly. Another explanation could be that the patient’s position, while measuring, deviated from the prescribed method of the manufacturer [11]. The manual states that measurements should be performed in a sitting position, with the elbow resting on a firm surface, and with the device hanging on the finger downwards below the heart level. This position was not attainable in the perioperative setting. Another possibility for the high malfunction rate could be the use of vasopressor therapy during surgery. In the majority of the malfunctions the patient received a vasopressor. The associated peripheral vasoconstriction could provide an explanation. The high malfunctioning rate of the device and mentioned possible explanation therefor make the TensorTip MTX unsuitable for use during surgery, especially when the hands of the patient are not easily accessible, which is commonly the case. The beat-to-beat measurement property of the device, put forward by the manufacturer was not realistic. View of the screen can be limited depending on its position and angle. The TensorTip MTX offers a solution for this last problem. With software and a downloadable application (app), all data can be read remotely and can also be viewed retrospectively. In this area, this device meets the expectations of future hemodynamic measurement equipment [21, 22].
We acknowledge several limitations to the study. Firstly, this is a single-center observational study, in which a limited number of subjects were included. Secondly, when calculating the group size, we underestimated the problem probability due to the error messages of the device. Unfortunately, the device eventually gave continuously error messages, and this made it impossible to continue using it, and to include more subjects. We recorded only one measurement of the reference method. Measurements were obtained during hemodynamic stable periods and minimal variation in BP was to be expected. Nevertheless, the averages of three TensorTip values have been compared to a single value of the reference method, artificially reducing the SD. We deliberately opted for one measurement moment per patient so that the measurements would not interfere with standard perioperative care. Consequently, calculating the trending ability was not possible, which ideally is investigated in a method comparison study [23]. We have used the automatic setting of the virtual arm cuff instead of setting the most suitable virtual arm cuff per patient. As a result, the device may not have taken the most sensitive measurements. A new study should show whether a difference in settings is reflected in the measurements.
Our study demonstrates that noninvasive BP measurements obtained with the noninvasive TensorTip MTX device are not equivalent to BP measurements with an invasive arterial catheter in the perioperative setting. Bias, SD, and therefore LoA and PE deviated too much from the suggested maximum values. The measurement performance was clinically not acceptable. Although the device has some advanced features, the high malfunction rate makes the device technically unsuitable for use in perioperative care at this time. Further research should demonstrate whether further technological developments will tackle the limitations that we have identified.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Parts of this study were presented at the ESAIC conference 2022.
Informed Consent
All patients gave written informed consent.
Author Contributions
SS: acquisition, analysis, and interpretation of data, writing and revising the manuscript, composition of tables and figures. LvE: analysis and interpretation of data, revising the manuscript critically. SdV: acquisition of data. IM: analysis and interpretation of data, revising the manuscript critically. CS: conception and design of the study, analysis, and interpretation of data, revising the manuscript critically
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
BP: blood pressure; SD: standard deviation; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; MBP: mean blood pressure; LoA: limits of agreement; PE: percentage error; CI: confidence interval
| References | ▴Top |
- Saugel B, Hoppe P, Khanna AK. Automated continuous noninvasive ward monitoring: validation of measurement systems is the real challenge. Anesthesiology. 2020;132(3):407-410.
doi pubmed - Turan A, Chang C, Cohen B, Saasouh W, Essber H, Yang D, Ma C, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. 2019;130(4):550-559.
doi pubmed - Saugel B, Dueck R, Wagner JY. Measurement of blood pressure. Best Pract Res Clin Anaesthesiol. 2014;28(4):309-322.
doi pubmed - Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109(6):1763-1781.
doi pubmed - Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, Frick G, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36(3):472-478.
doi pubmed - Meidert AS, Saugel B. Techniques for non-invasive monitoring of arterial blood pressure. Front Med (Lausanne). 2017;4:231.
doi pubmed - Segman YJ. New method for computing optical hemodynamic blood pressure. Journal of Clinical & Experimental Cardiology. 2016;7(12):1000492.
doi - Segman YJ, Sheiman E. Post marketing study of hemodynamic and hematological noninvasive readings in a blood bank. SAGE Open Med. 2018;6:2050312118796065.
doi pubmed - Segman YJ, Trahtemberg U. Oximeter behavior while using a tourniquet. Journal of Clinical & Experimental Cardiology. 2016;7(11):1000480.
doi - Saugel B, Grothe O, Nicklas JY. Error grid analysis for arterial pressure method comparison studies. Anesth Analg. 2018;126(4):1177-1185.
doi pubmed - CNOGA Medical Ltd., TensorTip MTX User's Guide. 2018. www.cnoga.com (Document#: MTX 1017).
- Viechtbauer W, Smits L, Kotz D, Bude L, Spigt M, Serroyen J, Crutzen R. A simple formula for the calculation of sample size in pilot studies. J Clin Epidemiol. 2015;68(11):1375-1379.
doi pubmed - Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307-310.
doi - Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15(2):85-91.
doi pubmed - Martina JR, Westerhof BE, van Goudoever J, de Beaumont EM, Truijen J, Kim YS, Immink RV, et al. Noninvasive continuous arterial blood pressure monitoring with Nexfin(R). Anesthesiology. 2012;116(5):1092-1103.
doi pubmed - Meidert AS, Huber W, Muller JN, Schofthaler M, Hapfelmeier A, Langwieser N, Wagner JY, et al. Radial artery applanation tonometry for continuous non-invasive arterial pressure monitoring in intensive care unit patients: comparison with invasively assessed radial arterial pressure. Br J Anaesth. 2014;112(3):521-528.
doi pubmed - Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160.
doi pubmed - Haghayegh S, Kang HA, Khoshnevis S, Smolensky MH, Diller KR. A comprehensive guideline for Bland-Altman and intra class correlation calculations to properly compare two methods of measurement and interpret findings. Physiol Meas. 2020;41(5):055012.
doi pubmed - Juri T, Suehiro K, Uchimoto A, Go H, Fujimoto Y, Mori T, Nishikawa K. Error grid analysis for risk management in the difference between invasive and noninvasive blood pressure measurements. J Anesth. 2021;35(2):189-196.
doi pubmed - Schumann R, Meidert AS, Bonney I, Koutentis C, Wesselink W, Kouz K, Saugel B. Intraoperative blood pressure monitoring in obese patients. Anesthesiology. 2021;134(2):179-188.
doi pubmed - Michard F, Scheeren TWL, Saugel B. A glimpse into the future of postoperative arterial blood pressure monitoring. Br J Anaesth. 2020;125(2):113-115.
doi pubmed - Scheeren TWL, Ramsay MAE. New developments in hemodynamic monitoring. J Cardiothorac Vasc Anesth. 2019;33(Suppl 1):S67-S72.
doi pubmed - Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111(5):1180-1192.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


