| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 2, April 2022, pages 88-96
Trends and Outcomes of Oral Anticoagulation With Direct Current Cardioversion for Atrial Fibrillation/Flutter at an Academic Medical Center
Samiullah Arshada, h , George A. Davisb, Muhammad Amirc, Ythan H. Goldbergd, Vedant A. Guptae, Ahmed K. Abdel-Latiff, Susan Smythg
aDepartment of Internal Medicine, University of Kentucky, Lexington, KY, USA
bPharmacy Services and College of Pharmacy, University of Kentucky, Lexington, KY, USA
cDepartment of Internal Medicine, National University of Medical Sciences, Islamabad, Pakistan
dDepartment of Cardiology, Montefiore Medical Center, Bronx, NY, USA
eDepartment of Cardiology, University of Kentucky, Lexington, KY, USA
fDepartment of Cardiology, University of Michigan, Ann Arbor and the Ann Arbor VA Healthcare System, MI, USA
gDepartment of Cardiology, University of Arkansas Medical Sciences, Little Rock, AZ, USA
hCorresponding Author: Samiullah Arshad, Department of Internal Medicine, University of Kentucky, Lexington, KY, USA
Manuscript submitted January 12, 2022, accepted February 15, 2022, published online March 12, 2022
Short title: Safe Use of DOACs for Cardioversion
doi: https://doi.org/10.14740/cr1352
| Abstract | ▴Top |
Background: Increasing reports suggest the safe use of direct oral anticoagulants (DOACs) in electrical cardioversion. The aim of this study was to assess the trends and 30-day outcomes associated with anticoagulation for cardioversion.
Methods: Patients who underwent electrical cardioversion from January 2015 to October 2020 with a 30-day follow-up were included; and outcomes including stroke, transient ischemic attack, intracranial hemorrhage (ICH), and major gastrointestinal bleeding were recorded.
Results: Of the 515 patients, 351 (68%) were men and 164 (32%) were women, with a mean CHA2DS2VASc score of 2.6 ± 1.6. Outpatient apixaban use increased from 10% in 2015 to 46% in 2020 (P < 0.001) with a decline in the use of warfarin from 24% in 2015 to 10% in 2020 (P = 0.023). Apixaban use peri-procedurally for cardioversion increased from 32% in 2015 to 35% in 2020 (P = 0.317), while warfarin use decreased from 23% in 2015 to 14% in 2020 (P = 0.164). At discharge, apixaban prescriptions increased from 21% in 2015 to 61% in 2020 (P < 0.001), while warfarin prescriptions declined from 30% in 2015 to 13% in 2020 (P = 0.009). No ICH was recorded in the 30 days after cardioversion. Ischemic stroke occurred in four (0.7%) patients with one (0.29%) of the 338 patients on a DOAC, one (0.8%) of the 124 patients on warfarin and two (5.5%) of the 36 patients not receiving anticoagulation post cardioversion. There were seven (1%) major gastrointestinal bleeding events in patients on oral anticoagulation, of which four (3%) were on warfarin and three (0.8%) were on DOACs.
Conclusions: Our study shows the increasing and safe use of DOACs for the purpose of cardioversion. The rates of 30-day ischemic stroke post cardioversion were low and only occurred in patients admitted in the intensive care unit.
Keywords: Anticoagulation; DOACs; VKA; Cardioversion; Trends; Outcomes
| Introduction | ▴Top |
The prevalence of atrial fibrillation has increased three-fold over the last 50 years [1]. Since its approval over 60 years ago, warfarin had been the main oral anticoagulant used in the USA for stroke prophylaxis in the setting of atrial fibrillation. Direct oral anticoagulants (DOACs) were first introduced into the USA clinical market in 2010 and have changed the spectrum of anticoagulation options available to patients. Their convenience of use, increasing reimbursement through insurance, higher compliance rates, and lower associated bleeding risk have been the driving factors for their use for stroke prophylaxis in atrial fibrillation. As a result, DOACs have been adopted rapidly and are increasingly being used for systemic embolic prevention in atrial fibrillation [2]. Their initiation immediately prior to or after cardioversion of atrial fibrillation is a logistically attractive approach. Direct current cardioversion carries an inherent risk of thromboembolism with the potential of dislodging a clot present in the left atrial appendage (LAA) after the return of sinus rhythm. Atrial stunning following cardioversion is also thought to lead to new thrombus formation [3]. The risk of thromboembolism approaches 7% in patients undergoing direct current cardioversion who are not on anticoagulation [4]. As highlighted in the RELY trial, with anticoagulation, risk of periprocedural thromboembolism surrounding cardioversion can be reduced to as low as 0.3% [5]. The EMANATE trial showed that apixaban was a feasible option for patients with atrial fibrillation of less than 48 h undergoing cardioversion, and rates of bleeding and thromboembolism were comparable to use of vitamin K antagonist (VKA) [6]. The X-VeRT trial concluded that rivaroxaban is an effective alternative to VKA for cardioversion [7]. A meta-analysis that included six randomized controlled trials on DOACs showed cardioversion of patients on DOACs had similar risks of a major ischemic event, bleeding, and mortality in comparison to warfarin [8].
Our institution initiated the UK Health Care OptimalCare™ anticoagulation guidelines (Supplementary Material 1, www.cardiologyres.org) to outline pre, peri- and post-procedural anticoagulation for cardioversion in line with the American College of Cardiology/American Heart Association recommendations. For atrial fibrillation of greater than 48 h duration or unknown time of onset, an early cardioversion strategy recommended includes transesophageal echocardiography (TEE) done to rule out LAA thrombus, following which, cardioversion can be performed in 2 h after first dose of DOAC, or 4 h after low-molecular-weight heparin (LMWH) or 24 h of unfractionated heparin (UFH), while the conventional strategy consists of anticoagulation for at least 3 weeks with DOAC or warfarin (goal international normalized ratio (INR): 2 - 3), followed by cardioversion, without the need for TEE. All patients with atrial fibrillation of greater than 48 h duration are to be anticoagulated for at least 4 weeks. For atrial fibrillation with duration of less than 48 h, initiation of anticoagulation can be followed by cardioversion after either 2 h of the first dose of DOAC or 4 h of LMWH or 24 h of UFH. For patients with hemodynamic instability, initiation of anticoagulation with immediate cardioversion without delay, followed by at least 4 weeks of anticoagulation is recommended. The aim of our study was to assess the trends of anticoagulation use at the time of direct current cardioversion and its impact on 30-day outcomes.
| Materials and Methods | ▴Top |
A retrospective review of medical charts of patients who underwent direct current cardioversion from January 2015 to October 2020 was performed. The study was approved by the Institutional Review Board of the University of Kentucky (UK, protocol number 63114). UK Healthcare provides quaternary care services to a large population of patients in Kentucky, West Virginia and Ohio. Patients were identified through the Center for Clinical and Translation Science’s (CCTS) electronic data warehouse. Charts were reviewed to document the index direct current cardioversion for atrial fibrillation/flutter during the study duration. Baseline demographics including age, gender, body mass index (BMI), medical history, CHA2DS2VASc, cardioversion procedure note, transesophageal echocardiogram (TEE) for presence of LAA thrombus prior to cardioversion, outpatient, peri- and post-procedure anticoagulation were recorded. Patients with at least 30 days follow-up available in the electronic medical record were included in the study, resulting in a sample size of 515 patients (Fig. 1). Patients without a follow-up after the cardioversion were excluded from the study. Trends of anticoagulant use over the period of 6 years were noted. Primary clinical end points for the study were ischemic stroke or transient ischemic attack (TIA), intracranial hemorrhage, and major gastrointestinal bleeding within 30 days of cardioversion. Ischemic stroke/TIA was diagnosed with help of brain imaging and clinical assessment of neurological deficit. Major bleeding was defined per the International Society on Thrombosis and Hemostasis as loss of two units of bloods, requiring transfusion or bleeding at a critical site.
 Click for large image | Figure 1. Study flowchart. TIA: transient ischemic attack. |
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Statistical analysis
Mean and standard deviation (SD) were used to represent continuous variables with frequencies and percentages to represent categorical variables. Chi-square test (or Fisher’s exact test, as appropriate) was used to compare patients based on the anticoagulant group. A two-sided Z test was conducted to assess the significance of changes in use of each anticoagulant from 2015 to 2020. SPSS 26 was used to analyze data, and statistical significance was considered when the P value was less than 0.05.
| Results | ▴Top |
Of the 515 patients identified, 351 (68%) were men and 164 (32%) were women. The mean age of patients undergoing direct current cardioversion was 62 ± 13 years, with a range of 18 - 95 years. Mean CHA2DS2VASc of the group was 2.6 ± 1.6. Demographics for the cohort are presented in Table 1.
 Click to view | Table 1. Baseline Characteristics of Patients |
Aspirin was an outpatient medication for 142 (27%) and clopidogrel for 19 (4%) patients. Concurrent use of an antiplatelet agent with an anticoagulant occurred in 134 (26%) patients and triple therapy with two antiplatelet agents and an anticoagulant in seven (1%) patients.
The indication for direct current cardioversion was atrial fibrillation in 324 (63%) patients and atrial flutter in 191 (37%) patients. A TEE to assess for LAA thrombus prior to direct current cardioversion was performed in 210 (41%) patients, of which nine (4%) patients had a thrombus identified in the LAA with their cardioversion re-scheduled and performed after 12 weeks of outpatient anticoagulation. New onset atrial fibrillation of less than 48 h duration was identified in 34 (7%) patients, with the vast majority of the patients in atrial fibrillation for greater than 48 h. Cardioversion was successful at restoring normal sinus rhythm in 448 (87%) patients. Emergent cardioversion was performed in 23 (4%) patients due to hemodynamic instability. Cardioversions were performed as an inpatient procedure (including those in the emergency department) in 322 patients (62%), of which 65 (12%) patients were in a medical or surgical intensive care unit (ICU). Ambulatory elective cardioversions were done for 193 (38%) patients.
Three hundred thirty (64%) patients were on anticoagulation for at least 30 days prior to cardioversion. As the baseline anticoagulant that patients were on in the community prior to presentation, apixaban was the most common anticoagulant in 146 (28%) patients followed by warfarin in 101 (20%) and rivaroxaban in 79 (15%) patients. Apixaban use increased from 10% in 2015 to 46% in 2020 (P < 0.001), rivaroxaban use did not change significantly over the 6 years (P = 0.981), and warfarin use declined from 24% in 2015 to 10% in 2020 (P = 0.023) (Fig. 2a). DOACs use prior to admission in 2020 was noted in 42 (45%) of 72 patients, while in 2015 only 16 (22%) patients were on DOACs. The rise in outpatient DOAC use as a baseline anticoagulant also resulted in a subsequent decrease in the use of intravenous/subcutaneous anticoagulants (other anticoagulants) peri-procedurally (Fig. 3).
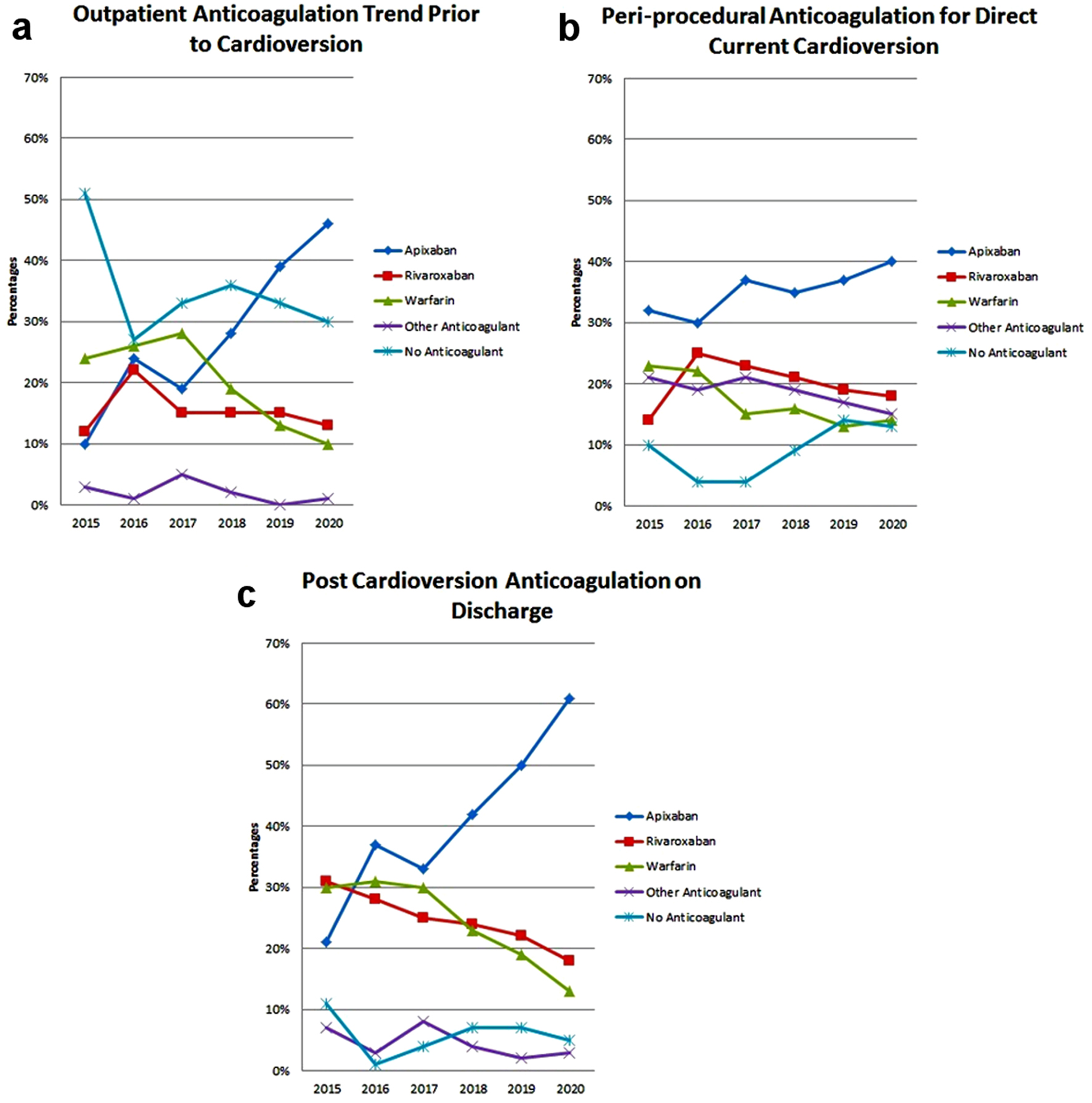 Click for large image | Figure 2. Trends in the use of oral anticoagulation from 2015 to 2020 in patients undergoing direct current cardioversion. (a) Use of anticoagulants for 30 days or more prior to cardioversion. (b) Anticoagulation use at the time of cardioversion. (c) Anticoagulation on discharge after cardioversion. |
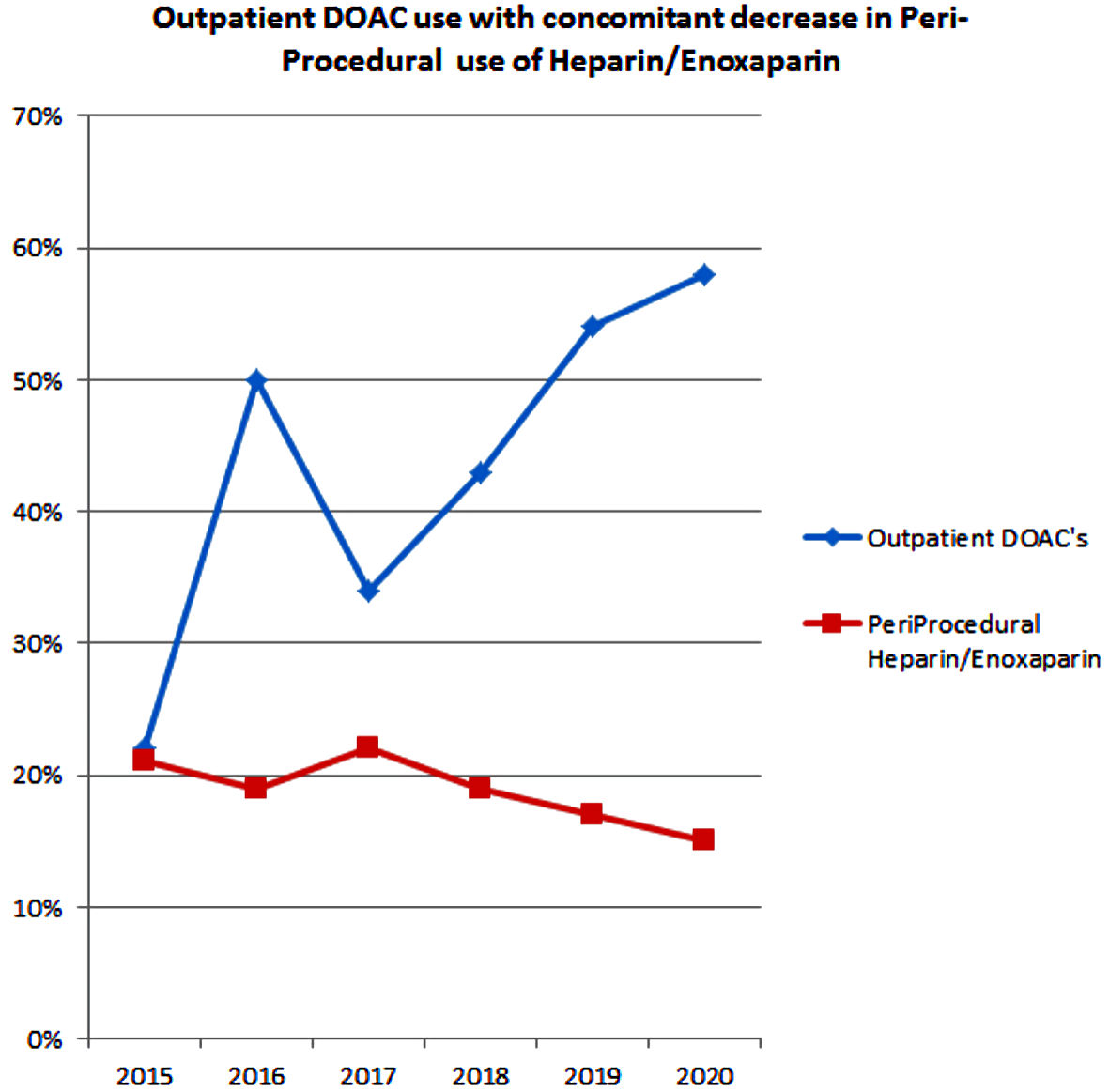 Click for large image | Figure 3. Declining intravenous/subcutaneous anticoagulation during cardioversion with rise of DOACs over 2015 - 2020. DOACs: direct oral anticoagulants. |
At the time of cardioversion, apixaban (35%), rivaroxaban (20%) and warfarin (17%) were the most common oral anticoagulants in the overall cohort. Other anticoagulants (including UFH, enoxaparin and bivalirudin) were used in 19% of patients. Only 9% of patients did not receive anticoagulation at the time of cardioversion; and of these, 3% had atrial fibrillation/flutter for 48 h or less. While there were trends of change in the use of anticoagulants at the time of cardioversion, none were statistically significant (Fig. 2b).
At the time of discharge, 212 (41%) patients were prescribed apixaban, 126 (25%) patients were prescribed rivaroxaban and 124 (24%) patients were prescribed warfarin. Thirty-one patients (6%) were discharged without any anticoagulant for the reasons listed in Table 2. After discharge, anticoagulation was continued for more than 30 days in 419 (81%) patients and for 30 days only in 51 (10%) patients who had CHA2DS2VASc scores of less than 2. Apixaban use at discharge increased significantly from 21% in 2015 to 61% in 2020 (P < 0.001), while warfarin use decreased from 30% in 2015 to 13% in 2020 (P = 0.009). There was a trend towards a decline in the prescription of rivaroxaban post cardioversion, from 31% in 2015 to 18% in 2020 (P = 0.068) (Fig. 2c).
 Click to view | Table 2. Reasons for No Anticoagulation on Discharge After Cardioversion |
Among the subset of 322 hospitalized patients undergoing cardioversion, peri-procedurally DOAC use increased over the time frame studied. Apixaban was used in 121 (37%) of these 322 patients followed by rivaroxaban in 68 patients (21%) and warfarin in 51 (16%) patients of the inpatient group. No anticoagulation was given to 42 (13%) patients, and 40 (12%) patients received other anticoagulants (intravenous/subcutaneous). At the time of discharge from the inpatient setting, 132 (41%) patients received apixaban, 65 (20%) received rivaroxaban, 76 (23%) received warfarin, 31 (10%) patients were discharged without anticoagulation and 18 (5%) patients continued other anticoagulants (intravenous/subcutaneous anticoagulants).
A comparison of baseline characteristics of patients who were discharged on warfarin or DOACs (apixaban and rivaroxaban) post cardioversion is described in Table 3. The warfarin group comprised a more complex patient population with a higher proportion of patients with chronic kidney disease (CKD) stage 3 - 5, ejection fraction (EF) < 40%, history of venous thromboembolism, previous major bleeding, higher concomitant use of aspirin, clopidogrel and triple therapy (defined as two antiplatelet agents with an oral anticoagulant).
 Click to view | Table 3. Patient Demographics Based on Type of Post-Procedural Anticoagulation |
At 30 days of follow-up, ischemic stroke occurred in four (0.7%) patients. All four patients who had a stroke were in the ICU, with one (0.29%) patient on rivaroxaban of the 338 patients on DOACs, one (0.8%) of 124 patients on warfarin, and two (5.5%) of the 36 patients who did not receive anticoagulation after cardioversion (Table 4). No intracranial hemorrhage occurred. There were seven (1%) major gastrointestinal bleeding events recorded in patients on oral anticoagulation in the 30 days follow-up period, of which four (3%) of 124 patients were on warfarin and three (0.8%) of 338 patients were on DOACs. Of these three patients who had major gastrointestinal bleeding on DOACs, two were on apixaban, and one on rivaroxaban.
 Click to view | Table 4. Characteristics of Patients With Stroke After Direct Current Cardioversion |
| Discussion | ▴Top |
Data from our institution demonstrates that DOACs have become the preferred anticoagulant for cardioversion, with 79% of patients post cardioversion receiving a DOAC in 2020. The majority (94%) of patients were anticoagulated for at least 30 days after cardioversion, indicating compliance with the OptimalCare™ Pathway. The rate of use of triple therapy was low and in line with safe practice.
Our study adds to the real-world data about the use of DOACs for cardioversion. The baseline characteristics of our patients are similar to those reported by two other real-world studies on DOACs use for cardioversion by De Heide et al [9] and Frederiksen et al [10], with the mean CHA2DS2VASc score of patients undergoing cardioversion being 2.3 and 2.5 respectively. The group of patients taking VKAs described by De Heide et al [9] also exhibited comparable clinical complexity to our patient population with a higher proportion of patients with congenital heart disease, congestive heart failure, coronary heart disease, diabetes mellitus, left ventricular dysfunction and renal insufficiency. Heart failure and left atrial enlargement were predominant in patients on VKA in the study by Frederiksen et al [10]. In our analysis, patients on warfarin therapy had higher rates of CKD, heart failure with reduced EF, a previous history of venous thromboembolism, and previous major bleeding. In the study by De Heide et al [9], the use of peri-procedural DOACs increased from 17% in 2015 to 73% in 2020, and an uptrend of peri-procedural DOACs is seen in our study. While in their study, dabigatran was the primary DOAC, our study showed predominant use of apixaban. Rates of ischemic stroke were comparable to our study results with 0.15% in DOAC group reported in the study by Frederiksen et al [10], and 0.41% in DOAC group reported in De Heide et al [9]. Neither of those studies had a group of patients without anticoagulation post cardioversion. No intracranial hemorrhage was reported in the study of Frederiksen et al [10], while one patient had a trauma associated subdural hematoma in the study by De Heide et al [9].
Our study is unique in that it encompasses direct current cardioversions performed in both inpatient and outpatient setting including emergent cardioversions and cardioversion of patients admitted in the ICU, while previous studies focused on cardioversion performed electively [11, 12].
The increased use of DOACs may be explained in part by greater clinician comfort in terms of pharmacologic response, ease of use for patients without the need for laboratory monitoring, access to new reversal agents, and better coverage of DOACs cost by prescription insurance. In a survey of Medicare D patients from 2011 to 2019, the proportion using DOACs increased from 7.4% in 2011 to 66.8% in 2019 [13]. In 2019, per this survey, the three most commonly used medications were apixaban (41.4%), warfarin (29.6%), and rivaroxaban (21.6%). A recent study of Department of Defense patient population with non-valvular atrial fibrillation indicated that patients on warfarin incurred higher all-cause health care costs per patient per month (PPPM) ($2,498 vs. $2,277; P = 0.148), significantly higher stroke/systemic embolism (SE)-related ($118 vs. $46; P = 0.012) and major bleeding-related ($166 vs. $76; P = 0.003) medical costs compared to apixaban [14]. Studies from several other countries have shown the cost effectiveness of apixaban over other DOACs and warfarin for stroke prevention in atrial fibrillation [15-19]. Lower risk of major bleeding and ischemic stroke with use of apixaban in comparison to rivaroxaban adds to the increasing popularity of apixaban for thromboprophylaxis in atrial fibrillation/flutter [20, 21]. Current data suggest use of DOACs is associated with a shorter wait time to cardioversion compared to warfarin [9] and fewer procedures canceled compared to warfarin, which also points towards cost effectiveness.
Compared to the 30% of patients in the entire cohort with coronary artery disease and atrial fibrillation, 26% of patients were on combination therapy with an anticoagulant and an antiplatelet agent, with aspirin being the primary antiplatelet agent. OAC-ALONE [22] and AFFIRE [23] challenged the notion of using combination antiplatelet with anticoagulant therapy in patients with atrial fibrillation and stable coronary artery disease. However, the practice pattern suggested by the percentages of use of combination therapy in our study indicates our clinicians opt for the approach of weighing the risk of bleeding with the benefit of preventing myocardial ischemic event.
Patients admitted in the ICU are at a higher risk of stroke, with an incidence approaching 1-2% admitted for non-neurological causes. Of the 65 patients admitted in the intensive care setting, four (6%) had an ischemic stroke and 20% were not on anticoagulation. Hypotension, arrhythmia burden, coagulation imbalance, surgery and pro-inflammatory states all increase risk of stroke [24, 25]. Further, cardioversions done for arrhythmias causing hemodynamic instability may not be effectively preceded by cardiac imaging to rule out intracardiac thrombus. Anticoagulation rates in the ICU remain low; with the AFTER-ICU study noting 60% patients received no anticoagulation in the ICU for atrial fibrillation [26]. All the ischemic strokes in our study occurred in patients admitted to ICU who had recent surgery or hemodynamic compromise and were not preceded by a TEE.
Study limitations
Our study has several limitations. Our sample size was reduced from initially screened 957 patients down to 515 patients due to a lack of 30-day follow-up, as the excluded 442 patients returned to follow-up with their primary cardiologist after cardioversion, and follow-up data for these patients was not available for review. Our study did not compare the time to cardioversion using different anticoagulants, nor did our study account for canceled procedures. Our study did not assess minor bleeding, drug interaction and changes in oral anticoagulants. In addition, adherence to DOACs was not assessed in our study. Due to the small number of primary end events, a statistical comparison between patients on DOACs and warfarin for ischemic stroke and intracranial hemorrhage could not be performed.
Conclusions
Our single-center study shows the increasing use of DOACs both for atrial fibrillation and flutter and for the purpose of cardioversion. Apixaban was the most prescribed anticoagulant. Ninety-one percent of patients received anticoagulation post cardioversion for at least 30 days. The rates of 30-day ischemic stroke post cardioversion were low and only occurred among patients admitted in the ICU. Further studies are needed to assess the use of anticoagulation and the risk of stroke post cardioversion in patients admitted in the ICU.
Learning points
Use of DOACs increased from 2015 to 2020 for outpatient anticoagulation and peri-procedurally for electrical cardioversion of atrial fibrillation/flutter, with a decrease in use of warfarin. Patients on warfarin were likely to have CKD, left ventricular ejection fraction (LVEF) < 40%, history of venous thromboembolism and history of major bleeding. Stroke occurred in four (0.7%) patients within 30 days after cardioversion and no intracranial hemorrhages were noted. All four patients were admitted in the ICU. Further studies are needed to assess trends of anticoagulation and outcomes of electrical cardioversion for atrial flutter/fibrillation among patients in the ICU.
| Supplementary Material | ▴Top |
Suppl 1. OptimalCare™ anticoagulation guidelines.
Acknowledgments
None to declare.
Financial Disclosure
This study was not funded.
Conflict of Interest
None of the authors has any conflict of interest to declare.
Informed Consent
No consent was required for this study as it was retrospective chart review.
Author Contributions
SS conceptualized the study idea. SA extracted and analyzed the data. The initial manuscript was drafted by SA, GD and SS. All authors contributed to the critical review and improvement of the manuscript and have reviewed the final version of the manuscript prior to submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
DOACs: direct oral anticoagulants; VKA: vitamin K antagonist; DC: direct current; CKD: chronic kidney disease; TEE: transesophageal echocardiogram; TIA: transient ischemic attack; AC: anticoagulation; LAA: left atrial appendage
| References | ▴Top |
- Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154-162.
doi - Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. 2015;128(12):1300-1305.e1302.
doi pubmed - Fatkin D, Kuchar DL, Thorburn CW, Feneley MP. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for "atrial stunning" as a mechanism of thromboembolic complications. J Am Coll Cardiol. 1994;23(2):307-316.
doi - Bjerkelund CJ, Orning OM. The efficacy of anticoagulant therapy in preventing embolism related to D.C. electrical conversion of atrial fibrillation. Am J Cardiol. 1969;23(2):208-216.
doi - Nagarakanti R, Ezekowitz MD, Oldgren J, Yang S, Chernick M, Aikens TH, Flaker G, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123(2):131-136.
doi pubmed - Ezekowitz MD, Pollack CV, Jr., Halperin JL, England RD, VanPelt Nguyen S, Spahr J, Sudworth M, et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J. 2018;39(32):2959-2971.
doi pubmed - Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, Talajic M, et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35(47):3346-3355.
doi pubmed - Telles-Garcia N, Dahal K, Kocherla C, Lip GYH, Reddy P, Dominic P. Non-vitamin K antagonists oral anticoagulants are as safe and effective as warfarin for cardioversion of atrial fibrillation: A systematic review and meta-analysis. Int J Cardiol. 2018;268:143-148.
doi pubmed - de Heide J, de Wit A, Bhagwandien RE, Assaf A, Gros-Bisdom J, van der Meer KC, Wijchers SA, et al. Efficacy and safety of direct oral anticoagulants in patients undergoing elective electrical cardioversion: A real-world patient population. Int J Cardiol. 2021;326:98-102.
doi pubmed - Frederiksen AS, Albertsen AE, Christesen AMS, Vinter N, Frost L, Moller DS. Cardioversion of atrial fibrillation in a real-world setting: non-vitamin K antagonist oral anticoagulants ensure a fast and safe strategy compared to warfarin. Europace. 2018;20(7):1078-1085.
doi pubmed - Itainen-Stromberg S, Hekkala AM, Aro AL, Vasankari T, Airaksinen KEJ, Lehto M. Real-life experience with non-vitamin K antagonist oral anticoagulants versus warfarin in patients undergoing elective cardioversion of atrial fibrillation. Ann Noninvasive Electrocardiol. 2020;25(5):e12766.
doi pubmed - Hellman T, Kiviniemi T, Nuotio I, Vasankari T, Hartikainen J, Lip GYH, Airaksinen KEJ. Intensity of anticoagulation and risk of thromboembolism after elective cardioversion of atrial fibrillation. Thromb Res. 2017;156:163-167.
doi pubmed - Troy A, Anderson TS. National trends in use of and spending on oral anticoagulants among US medicare beneficiaries from 2011 to 2019. JAMA Health Forum. 2021;2(7):e211693.
doi - Gupta K, Trocio J, Keshishian A, Zhang Q, Dina O, Mardekian J, Rosenblatt L, et al. Real-world comparative effectiveness, safety, and health care costs of oral anticoagulants in nonvalvular atrial fibrillation patients in the U.S. Department of Defense Population. J Manag Care Spec Pharm. 2018;24(11):1116-1127.
doi pubmed - Lopes RD, Thomas L, Di Fusco M, Keshishian A, Luo X, Li X, Masseria C, et al. Clinical and economic outcomes among nonvalvular atrial fibrillation patients with coronary artery disease and/or peripheral artery disease. Am J Cardiol. 2021;148:69-77.
doi pubmed - Bowrin K, Briere JB, Levy P, Millier A, Tardu J, Toumi M. Real-world cost-effectiveness of rivaroxaban and apixaban vs VKA in stroke prevention in non-valvular atrial fibrillation in the UK. J Mark Access Health Policy. 2020;8(1):1782164.
doi pubmed - Walter E, Voit M, Eichhober G. Cost-effectiveness analysis of apixaban compared to other direct oral anticoagulants for prevention of stroke in Austrian atrial fibrillation patients. Expert Rev Pharmacoecon Outcomes Res. 2021;21(2):265-275.
doi pubmed - Oyaguez I, Suarez C, Lopez-Sendon JL, Gonzalez-Juanatey JR, de Andres-Nogales F, Suarez J, Polanco C, et al. Cost-effectiveness analysis of apixaban versus edoxaban in patients with atrial fibrillation for stroke prevention. Pharmacoecon Open. 2020;4(3):485-497.
doi pubmed - de Jong LA, Groeneveld J, Stevanovic J, Rila H, Tieleman RG, Huisman MV, Postma MJ, et al. Cost-effectiveness of apixaban compared to other anticoagulants in patients with atrial fibrillation in the real-world and trial settings. PLoS One. 2019;14(9):e0222658.
doi pubmed - Fralick M, Colacci M, Schneeweiss S, Huybrechts KF, Lin KJ, Gagne JJ. Effectiveness and safety of apixaban compared with rivaroxaban for patients with atrial fibrillation in routine practice: a cohort study. Ann Intern Med. 2020;172(7):463-473.
doi pubmed - Ray WA, Chung CP, Stein CM, Smalley W, Zimmerman E, Dupont WD, Hung AM, et al. Association of rivaroxaban vs apixaban with major ischemic or hemorrhagic events in patients with atrial fibrillation. JAMA. 2021;326(23):2395-2404.
doi pubmed - Matsumura-Nakano Y, Shizuta S, Komasa A, Morimoto T, Masuda H, Shiomi H, Goto K, et al. Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation. 2019;139(5):604-616.
doi pubmed - Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381(12):1103-1113.
doi pubmed - Pilato F, Profice P, Dileone M, Ranieri F, Capone F, Minicuci G, Tagliente D, et al. Stroke in critically ill patients. Minerva Anestesiol. 2009;75(5):245-250.
- Jo S, Chang JY, Jeong S, Jeong S, Jeon SB. Newly developed stroke in patients admitted to non-neurological intensive care units. J Neurol. 2020;267(10):2961-2970.
doi pubmed - Yoshida T, Uchino S, Sasabuchi Y, AFTER-ICU study group. Clinical course after identification of new-onset atrial fibrillation in critically ill patients: The AFTER-ICU study. J Crit Care. 2020;59:136-142.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


