| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 10, Number 6, December 2019, pages 331-335
Expedited Removal of a Radial Hemostatic Compression Device Following Cardiac Catheterization Is Safe and Associated With Reduced Time to Discharge
Mark K. Tuttlea, b, c, Noah Q. Haroiana, b, Lana F. Gavina, Cheryl A. Espositoa, Kalon K.L. Hoa, b
aCardiovascular Division, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
bHarvard Medical School, Boston, MA 02115, USA
cCorresponding Author: Mark K. Tuttle, Cardiovascular Division, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
Manuscript submitted September 22, 2019, accepted October 7, 2019
Short title: Rapid Radial Compression Device Removal
doi: https://doi.org/10.14740/cr953
| Abstract | ▴Top |
Background: Radial access for cardiac catheterization has become increasingly adopted, owing much of its popularity to decreased bleeding complications compared with the femoral approach. Hemostatic compression devices (HCDs) for radial catheterization play a key role in this advantage, but the optimal duration of compression is unknown. A shorter duration of compression is encouraged by guidelines, but removing an HCD too quickly could result in serious bleeding. We aimed to evaluate the safety and effectiveness of expedited removal of a radial HCD after cardiac catheterization.
Methods: We conducted a prospective study of patients undergoing radial cardiac catheterization and/or percutaneous coronary intervention at a tertiary care academic medical center. Patients underwent HCD application using a TR Band® (Terumo Interventional Systems) which was removed after a prespecified amount of time in each of three sequential temporal cohorts: 2-h, 1-h, or 0.5-h. Each patient was monitored for development of bleeding or hematoma and for serious complications.
Results: A total of 354 patients participated in our study, with similar numbers in each group. There was a greater rate of minor bleeding in the 0.5-h (12%) and 1-h (19%) groups compared with the 2-h group (8%), but there were no serious complications (need for surgical consultation, transfusion, or unplanned admission) in any group. The average time to discharge was shorter in the 0.5-h and 1-h groups compared with the 2-h group.
Conclusions: Deflating the radial HCD at 0.5 h is safe with no increase in the observed rate of major complications and is associated with reduced time to discharge after coronary angiography or percutaneous coronary intervention using the radial arterial approach.
Keywords: Cardiac catheterization; Artery; Radial; Bleeding; Angioplasty; Transluminal; Percutaneous coronary
| Introduction | ▴Top |
Transradial vascular access has become an increasingly adopted approach for cardiac catheterization in the USA, with use of radial arterial access among percutaneous coronary interventions (PCIs) rising in the National Cardiovascular Data Registry (NCDR®) CathPCI Registry from 10.9% in 2011 to 25.2% in 2014 to 39.5% in 2017 [1, 2]. One of the drivers behind this trend is the lower rate of bleeding complications with the radial approach compared with the femoral approach [3].
A key factor in the decreased bleeding rates associated with radial access is the use of hemostatic compression devices (HCDs) placed post-procedurally. However, despite their importance, the optimal duration of post-procedural compression with an HCD is unclear. One benefit of shorter of duration of compression is lower rates of radial artery occlusion [4], prompting expert consensus guidelines to advocate for this strategy [5]. If faster removal of an HCD leads to less time required for patients to be observed in the hospital, this could also result in decreased length of stay and cost savings. However, a shorter duration of hemostatic compression would be detrimental if it leads to increased rates of bleeding. Thus, the ideal duration of compression that balances these factors remains unclear.
An informal survey of North American cardiac catheterization laboratory policies that we conducted reflects this uncertainty, with institutional stipulated compression times varying from 10 min to 4 h. At the outset of this study, our institution’s cardiac catheterization laboratory protocol mandated application of an HCD (the TR Band® from Terumo Interventional Systems, Somerset, NJ) for 2 h. The Removal Guidelines from Terumo suggest compression for 1 - 2 h depending on the amount of heparin used during the procedure [6]. However, the brochure indicates that these guidelines are consensus opinion only. Because of the potential benefits of shorter duration hemostatic compression after radial approach cardiac catheterization and the lack of data to guide practice, we sought to ascertain whether an expedited HCD removal protocol would be safe to implement at our institution.
| Materials and Methods | ▴Top |
Study design
We conducted a prospective cohort study of consecutive patients undergoing coronary angiography, left heart catheterization and/or PCI via the radial artery approach at the Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts, aiming to ascertain the safety of expedited removal of an HCD post-procedure compared with our standard practice of 120 min of compression. Most patients undergoing cardiac catheterization and PCI procedures receive immediate post-procedure care (including HCD or femoral arterial sheath management) in the Cardiac Catheterization Laboratory Holding Area. Patients with radial arterial access were excluded from this study if they were to be transferred from our holding area to an inpatient care area (intensive care unit or cardiology ward) prior to removal of the HCD. This ensured that our participants’ HCDs would be managed and removed exclusively by our catheterization laboratory holding area nursing staff who were educated on our study design and its data collection form. Patients were also excluded if they had a hematoma prior to HCD application, or if they were felt to be at excessive bleeding risk in the opinion of the interventional cardiologist who performed the procedure. Data collection took place from January 2017 through October 2017. This study was conducted under the supervision of our Cardiac Catheterization Laboratory Quality Improvement Committee and was approved by the BIDMC Institutional Review Board as a quality improvement initiative.
All participants underwent HCD application with a TR Band® as the arterial sheath (most often a 10-cm 6 French GlideSheath Slender®, Terumo Interventional Systems, Somerset, NJ) was removed in adherence with the device’s instructions for use provided by the manufacturer [6] with inflation of the device’s air cushion until hemostasis was achieved. After application of the HCD, participants were transferred to our post-procedure holding area and, if needed, immediately had air removed from the HCD cushion until patent hemostasis was achieved, as determined using Barbeau’s test [7]. Following a pre-defined duration of compression (120 min, 60 min, or 30 min), the HCD cushion was weaned off by removing one-third of the air content at three 15-min intervals, constituting phases 1 - 3 of our weaning protocol. Patients were subsequently observed for 15 min with the TR Band® in place, but completely deflated, after which time it was removed. Following removal of the TR Band®, patients were observed for a specified interval before being eligible for discharge. In the 2-h and 1-h groups, the post-removal observation interval was 30 min, and for the 0.5-h group, the post-removal observation time was 60 min (Fig. 1). The patients were then observed for a period of time in the holding area before being discharged home or transferred to an inpatient care area. If any bleeding occurred during cushion deflation, re-inflation with an additional 1 - 2 mL of air was performed until hemostasis was achieved, and the weaning period was extended by an additional 15 min interval. If any new hematoma was evident during cushion deflation, manual pressure was applied, and a cardiology fellow was called to assess the patient and determine subsequent management.
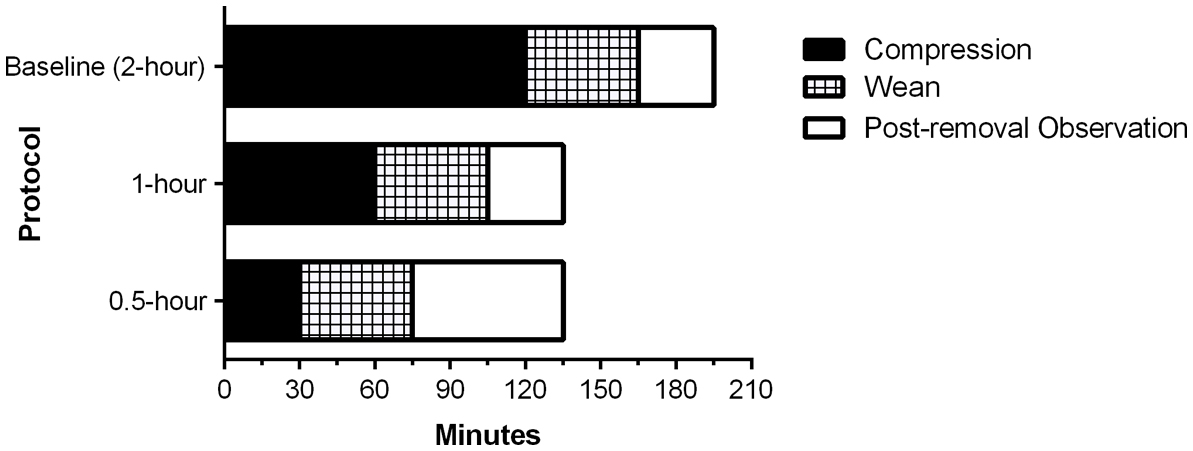 Click for large image | Figure 1. Compression device removal protocol times. This bar graph shows the compression, weaning, and post-removal observation times for patients in our study protocol in each of the three groups studied. |
Data collection
For each participant, we recorded basic demographic information, the type of procedure performed (diagnostic coronary angiography only, PCI, or other) and what anticoagulant was used. If an activated clotting time (ACT) was performed as part of PCI or to guide safe removal of sheaths at additional access sites, we recorded the time of measurement and the value. Timestamps were recorded for initial application of the HCD, each phase of deflation of the HCD cushion, the time of removal of the HCD, and the time of discharge or transfer. If any bleeding or hematoma occurred, the time of this event was also recorded. Lastly, we recorded occurrence of large hematoma, severe discomfort, transfusion, surgical consult or unplanned admission for radial arterial access site complications.
Using the above protocol, we collected data in three sequential stages. During stage 1 (January through February, 2017), we utilized our pre-existing standard timeframe of 2 h of compression prior to deflation of the HCD cushion in order to ascertain our baseline level of events. During stage 2 (February through April, 2017), we used a time of compression of 30 min. During stage 3 (September through October, 2017), we used 1 h. The weaning protocol was identical in all stages. However, post-weaning observation differed; post-weaning observation was mandated as at least 60 min in the 0.5-h weaning group and 30 min in the 1-h weaning group to ensure patient safety since this degree of rapid removal had not previously been studied in this manner. See the protocol (Supplementary Material 1, www.cardiologyres.org) for our data collection form for stage 3 (0.5-h group), which is similar to the other stages’ data collection forms.
Statistical methods
We performed statistical analyses using JMP/13 software (SAS Institute Incorporated, Cary, North Carolina). For patient characteristics and outcome events, proportions were compared using Fisher’s exact tests, and continuous variables were compared using analysis of variance and t-tests. Elapsed time variables were compared using Wilcoxon tests and the Kruskal-Wallis test.
| Results | ▴Top |
A total of 354 patients were included in our study; there were 99 patients in the 2-h weaning group, 132 patients in the 1-h weaning group, and 123 patients in the 0.5-h weaning group. Baseline demographic and clinical characteristics are shown in Table 1 and were similar across all three groups. Notably, there were no differences in proportion of PCI, heparin use, or mean ACT (if performed) across the three groups.
 Click to view | Table 1. Baseline Characteristicsa |
As shown in Table 2, the rate of bleeding in the baseline 2-h weaning group (8%) was lower than in the 1-h weaning group (19%, P = 0.01), and was not significantly different than in the 0.5-h weaning group (12%, P = 0.08.) There was no statistically significant difference in the rate of hematoma alone or the composite rate of bleeding or hematoma across all three groups. Notably, the serious complications endpoint (a composite of severe discomfort, severe hematoma, need for blood transfusion, surgical consultation, or unplanned admission) did not occur in any of the three groups.
 Click to view | Table 2. Outcomes by Group |
We theorized that minor bleeding events occurring early during the weaning process were less dangerous than bleeding events occurring late in the weaning process or after removal of the HCD, since the closer a bleeding event occurred to a patient’s discharge, the greater the risk another bleeding event could occur after discharge and be unable to be immediately acted upon by medical personnel. For this reason, we analyzed our data as depicted in Figure 2 and grouped bleeding and hematoma events by the phase of weaning during which they occurred among our three groups. Reassuringly, only two total bleeding events occurred after the HCD was removed; both of these events were adjudicated with manual medical record review and it was discovered that in both cases, the patients had developed bleeding immediately after using the bathroom, and it is suspected that they may have been nonadherent to the prescribed range of motion and activity restrictions for their involved wrist.
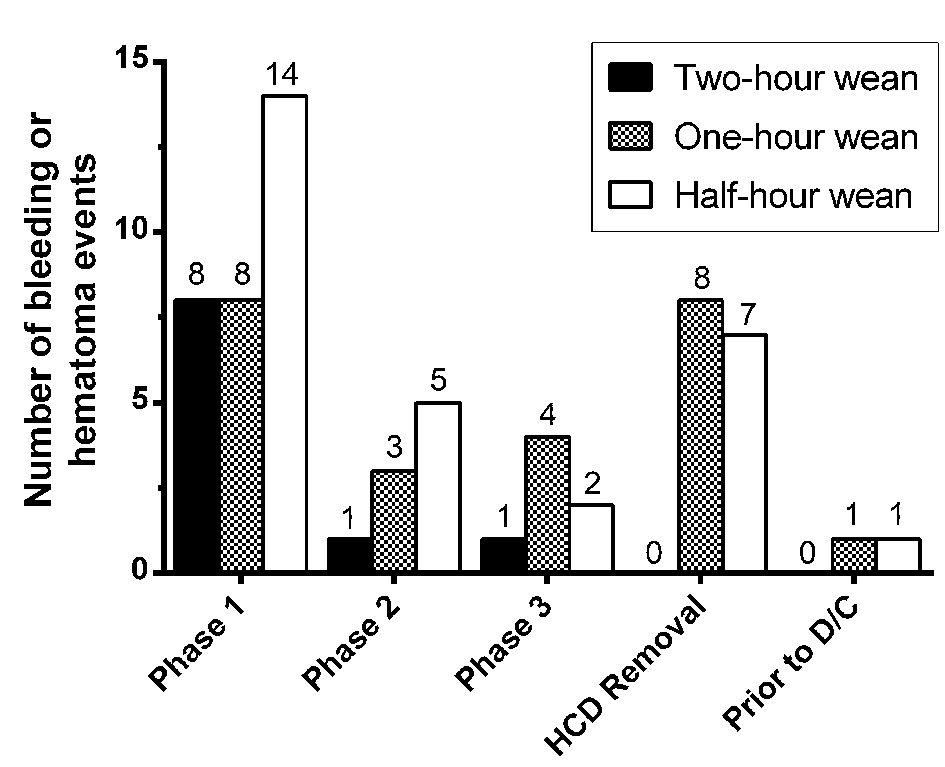 Click for large image | Figure 2. Bleeding and hematoma events grouped by weaning phase. As described in the methods section, hemostatic compression devices (HCDs) were removed with three serial deflations of one-third of the volume of air in the HCD cushion, 15 min apart, constituting phase 1 - 3 of the weaning protocol. Bleeding or hematoma could be noted during each of these phases, once the HCD was physically removed, or after the HCD was removed but before the patient was discharged. |
The time from HCD application to discharge was shorter in the 0.5-h weaning group (median: 3.0 h, P < 0.01) and 1-h weaning group (median: 3.0 h, P < 0.001) compared with the baseline 2-h weaning group (median: 3.5 h.) There was no significant difference in time to discharge between the 0.5-h weaning group and the 1-h weaning group (Fig. 3).
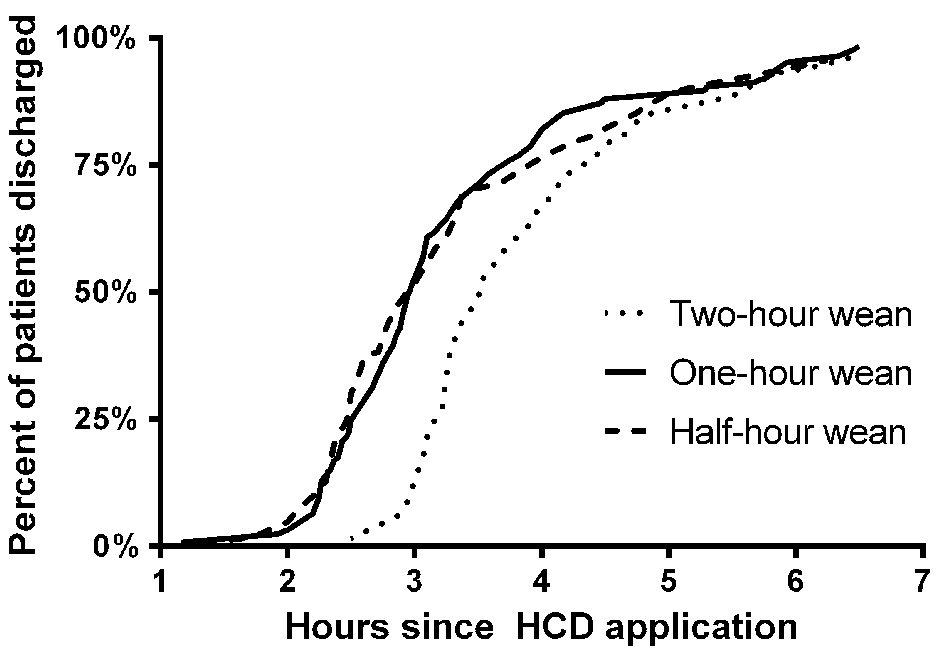 Click for large image | Figure 3. Time to discharge from HCD application. These cumulative frequency distributions illustrate how much time elapsed between hemostatic compression device (HCD) application and discharge for each of the three groups evaluated. Patients in the 1-h and 0.5-h weaning groups were discharged sooner than in the 2-h weaning group (P < 0.001 and P < 0.01, respectively, by Wilcoxon test). There was no significant difference between the 1-h and 0.5-h groups (P = NS by Wilcoxon test.) NS: not significant. |
Because anticoagulants and antiplatelet agents are used routinely during and after PCI procedures, and could contribute to bleeding or hematoma formation, we also examined these data in aggregate, detailed in Table 3. There were no significant differences in rates of bleeding or hematoma across diagnostic coronary angiography, PCI, or other cardiac catheterization procedures.
 Click to view | Table 3. Rates of Bleeding/Hematoma Stratified by Procedure Type |
| Discussion | ▴Top |
Although Carrington et al previously performed a similar analysis of accelerated removal of an HCD following cardiac catheterization [8], to our knowledge, our study is unique in that it included patients undergoing PCI and utilized modern patent hemostasis technique. Our study showed that reducing the duration of HCD application, although associated with increased rates of minor bleeding, was not associated with any increase in serious complications. The vast majority of bleeding and hematoma events occurred during serial deflations of the HCD air cushion, and were thus immediately recognized and abated with prompt re-inflation of the air cushion and waiting an additional 15 min before continuing; only four patients experienced recurrent bleeding (1%.)
Of note, the lack of a difference in time to discharge between the 1-h compression group and the 0.5-h compression group (Fig. 3) is likely explained by the different mandated post-removal observation times. In the 1-h group, patients were mandated to be observed for at least 30 min after HCD removal prior to discharge. In the 0.5-h group, this interval was 60 min. This longer interval was chosen to ensure patient safety and may explain the lack of a difference between discharge times of the 0.5-h and 1-h compression groups.
Our results confirm that reducing the duration of HCD application is associated with expedited time to discharge. Reducing time to discharge is beneficial for a number of reasons provided it is safe, particularly if it improves patient satisfaction and reduces healthcare costs by making a catheterization laboratory more efficient. A recent NCDR study showed that same-day discharge for PCI patients was associated with cost savings averaging $3,497 per procedure compared with non same-day discharge [9]. Although not evaluated in our study, an additional expected benefit of reduced duration of HCD compression is a decreased rate of radial artery occlusion [4]. It is worth noting that we do not advocate for same-day discharge for all PCI patients; however, in appropriately selected patients this is a safe and less costly disposition.
Our study has several limitations. With roughly 100 participants in each of our three groups, our study was underpowered to detect small differences in event rates. The lack of randomization and blinding also limited definitive causal inference. However, these same characteristics enabled us to perform the study relatively quickly and with limited resources. As a result, our hospital has implemented a 0.5-h weaning protocol for all transradial cardiac catheterization procedures. In post-implementation surveillance, as was the case during our study, there have been no serious complications. We hope that the reassuring results of this study will serve as a pilot for a more definitive randomized controlled trial.
Conclusions
Compared with 120 min of hemostatic device compression, an accelerated protocol of 60 min of compression or 30 min of compression yielded no serious complications in our cohort of patients, status post transradial cardiac catheterization. As expected, the accelerated protocols were also associated with reduced time to discharge.
| Supplementary Material | ▴Top |
Supplementary Material 1. Radial Artery TR Band Removal Protocol and Data Collection Tool.
Acknowledgments
We gratefully acknowledge the participation of our patients, the assistance of our cardiac catheterization laboratory physicians, and the invaluable contributions of the nursing and technologist staff in the cardiac catheterization laboratory and especially in the holding area.
Financial Disclosure
The authors have no relevant financial disclosure to declare.
Conflict of Interest
None to declare.
Informed Consent
Not required (quality improvement project with need for consent waived by the Institutional Review Board).
Author Contributions
MKT contributed to conception, study design, data collection, analysis, writing and revising the manuscript; NQH contributed to data collection and revising the manuscript; LFG contributed to conception, study design, data collection, analysis, and revising the manuscript; CAE contributed to data collection and revising the manuscript; KLH contributed to conception, study design, data analysis, and revising the manuscript.
| References | ▴Top |
- Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, Moore JWM, et al. Trends in U.S. Cardiovascular Care: 2016 Report From 4 ACC National Cardiovascular Data Registries. J Am Coll Cardiol. 2017;69(11):1427-1450.
doi pubmed - Cath PCI Registry Institutional Outcomes Report 2017 Q4. Washington, DC: National Cardiovascular Data Registry, 2018 (used with permission from the American College of Cardiology).
- Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44(2):349-356.
doi pubmed - Pancholy SB, Patel TM. Effect of duration of hemostatic compression on radial artery occlusion after transradial access. Catheter Cardiovasc Interv. 2012;79(1):78-81.
doi pubmed - Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff AR, Crisco V, et al. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention's transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236.
doi pubmed - Terumo Medical Corporation. TR Band Application and Removal Guidelines, 2017; http://www.terumois.com/content/dam/terumopublic/products/trband/TR-Band-Application-Guidelines.pdf, accessed 05/31/2018.
- Barbeau GR, Arsenault F, Dugas L, Simard S, Lariviere MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J. 2004;147(3):489-493.
doi pubmed - Carrington C, Mann R, El-Jack S. An accelerated hemostasis protocol following transradial cardiac catheterization is safe and may shorten hospital stay: a single-center experience. J Interv Cardiol. 2009;22(6):571-575.
doi pubmed - Amin AP, Patterson M, House JA, Giersiefen H, Spertus JA, Baklanov DV, Chhatriwalla AK, et al. Costs associated with access site and same-day discharge among medicare beneficiaries undergoing percutaneous coronary intervention: an evaluation of the current percutaneous coronary intervention care pathways in the United States. JACC Cardiovasc Interv. 2017;10(4):342-351.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


