| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 10, Number 2, April 2019, pages 83-88
Assessment of Mitral Annular Plane Systolic Excursion in Patients With Left Ventricular Diastolic Dysfunction
Dagmar F. Hernandez-Suareza, Francisco Lopez-Menendezb, Abiel Roche-Limac, Angel Lopez-Candalesa, c, d
aCardiovascular Medicine Division, University of Puerto Rico School of Medicine, San Juan, PR, USA
bDivision of Cardiovascular Health and Diseases, University of Cincinnati College of Medicine, Cincinnati, OH, USA
cCenter for Collaborative Research in Health Disparities, University of Puerto Rico School of Medicine, San Juan, PR, USA
dCorresponding Author: Angel Lopez-Candales, Cardiovascular Medicine Division, University of Puerto Rico School of Medicine, Medical Sciences Building, PO Box 365067, San Juan, PR 00936-5067, USA
Manuscript submitted January 23, 2019, accepted February 25, 2019
Short title: Mitral Annular Motion and Diastolic Dysfunction
doi: https://doi.org/10.14740/cr837
| Abstract | ▴Top |
Background: Mitral annular plane systolic excursion (MAPSE) is a well-known surrogate measurement of left ventricular ejection fraction (LVEF) and prognostic factor for many cardiac conditions. However, little is known about its role in assessing LV diastolic function; we therefore sought to identify potential determinants of MAPSE in patients with LV diastolic dysfunction (LVDD).
Methods: Our echocardiographic database was queried for studies of patients with normal sinus. Patients were allocated into three groups: LVDD 0, LVDD 1 and LVDD 2 in accordance with LVDD stages recommended by the American Society of Echocardiography guidelines.
Results: A total of 107 echocardiographic studies were included in the study. The mean MAPSE was 1.22 ± 0.32 cm. Groups LVDD 0 (n = 23), LVDD 1 (n = 43), and LVDD 2 (n = 41) were significantly different in most of the studied variables. Particularly, MAPSE differed between the three groups (P < 0.01). A multiple regression analysis showed that age, LVEF and LV mass index were predictors of MAPSE instead of LVDD and left atrial measurements. Finally, a regression model was constructed to predict MAPSE in the studied group showing that age and LVEF explained a 46% of the MAPSE variation. A two-way contour plot was illustrated to ease the model interpretation.
Conclusions: Age and measures of LV systolic function correlated well with MAPSE. A simplified model to predict MAPSE based on age and LVEF is proposed. Additional studies are needed to examine the potential role of MAPSE in identifying symptoms and overall prognosis in LVDD patients.
Keywords: Echocardiography; Mitral annular plane systolic excursion; Diastolic dysfunction
| Introduction | ▴Top |
Mitral annular plane systolic excursion (MAPSE) represents a useful surrogate of left ventricular (LV) longitudinal function and has been long used as a complimentary measure to ejection fraction (EF) [1]. Additionally, MAPSE has been suggested as an important prognostic factor in the risk stratification for patients with atrial fibrillation, post-myocardial infarction and heart failure [2-5]. As a marker of longitudinal systolic function, it may have increased sensitivity over traditional methods of systolic performance such as EF, at times aiding in detection of early systolic dysfunction. Furthermore, good correlation with longitudinal strain measurements has been previously reported [6]. However, strain measurements are not only always infeasible, especially in patients with poor echocardiographic window, but also not widely available in all echocardiographic laboratories for day-to-day clinical studies.
Conceptually, the mitral annulus (MA) lies between two different structures (left atrium (LA) and LV). Therefore, it stands to reason that geometrical distortions in both chambers may affect the excursion of the mitral apparatus from base to apex. However, although many studies highlight the importance of MAPSE in the assessment of longitudinal systolic function [1], little is known about its role in LV diastolic dysfunction (LVDD) with or without associated reduced systolic function.
Our group has recently highlighted the relationship between MA ascent motion and changes in LA pressure [7]. However, whether this atrio-ventricular coupling was the result of chamber geometry or anatomic continuity was never determined. Hence, since MA is both anatomically and functionally related to this left atrio-ventricular dependence, we now sought to identify both clinical and echocardiographic determinants of MAPSE in patients with LVDD in view of recent data suggesting that MAPSE might be a promising tool in the stratification of LVDD in obese adults with normal left ventricular ejection fraction (LVEF) [8].
| Materials and Methods | ▴Top |
Study population
Study approval was obtained from the University of Cincinnati Institutional Board Review (Protocol # 12061302) and since this was a retrospective study, no informed consent was therefore needed. Data was collected over a 3-month period using a consecutive sample technique. Our echocardiographic database was queried for studies of patients with any indication of echocardiography in normal sinus rhythm with no ectopy or any other arrhythmia and no significant left-sided valvular disease. Also, M-mode and tissue Doppler imaging (TDI) of the MA were required from all individuals as well as a good acoustic window. Patients with stage 3 LVDD were excluded, as we considered that the significant LV systolic dysfunction observed in these patients could be an important cofounder in the assessment of possible associations between LVDD and MAPSE.
The studied population was allocated into three groups: LVDD 0, LVDD 1 and LVDD 2, in accordance with LVDD stages recommended by the American Society of Echocardiography guidelines [9].
Echocardiographic studies
Two-dimensional echocardiographic studies were performed using commercially available systems (Vivid 7 and 9; GE Medical Systems, Milwaukee, WI, USA). Images were obtained in the parasternal and apical views with the patient in the left lateral decubitus position and in the subcostal view with the patient in the supine position using a 3.5 MHz transducer. Standard two-dimensional, color, pulsed, and continuous-wave Doppler data were digitally acquired in gently held end-expiration, and saved in regular cine loop format for subsequent offline analysis.
Additionally, a complete spectral Doppler study was also performed to examine LV diastolic function in accordance with American Society of Echocardiography guidelines [9]. LV diastolic function was classified as normal (LVDD 0), impaired relaxation (LVDD 1), and pseudonormal pattern (LVDD 2). All echocardiographic parameters including LV volumes, LVEF, left atrial volume index, LV mass, MAPSE, and all Doppler variables used to determine LV diastolic function were measured as previously reported by our group [10, 11].
Statistical analysis
All echocardiographic parameters were calculated using the commercially available software Merge Cardio Workstation (Merge Healthcare). Baseline characteristics were compared among groups using Chi-square for categorical data and analysis of variance (ANOVA) with Bonferroni correction for continuous variables assuming equal variances. A simple linear regression test was used to determine the correlation between all measurements and MAPSE. Additionally, a stepwise multiple linear regression analysis was performed to determine which of the correlated parameters was the best predictor of maximal of MA motion. Finally, a linear regression model was obtained to determine the contribution of age and LVEF toward MAPSE and a two-way contour plot was illustrated to better understand such contribution. Reliability of these measurements has already been published [7]. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA), and P values < 0.05 were considered statistically significant.
| Results | ▴Top |
Study population
A total of 107 patients with echocardiographic studies performed for any clinical indication met inclusion criteria. They were divided into three groups based on left ventricular diastolic function: LVDD 0 with no diastolic dysfunction (n = 23), LVDD 1 with impaired LV relaxation (n = 43) and LVDD 2 with pseudonormal pattern (n = 41). Table 1 shows clinical and echocardiographic characteristics of the study population. Gender and body surface area (BSA) were the only studied variables that were not associated with the LVDD stage (P > 0.05). Moreover, the mean MAPSE was 1.22 ± 0.32 cm and one-way ANOVA revealed that MAPSE was significantly different between the three LVDD groups (P < 0.01) (Fig. 1).
 Click to view | Table 1. Demographic and Echocardiographic Data of the Study Population |
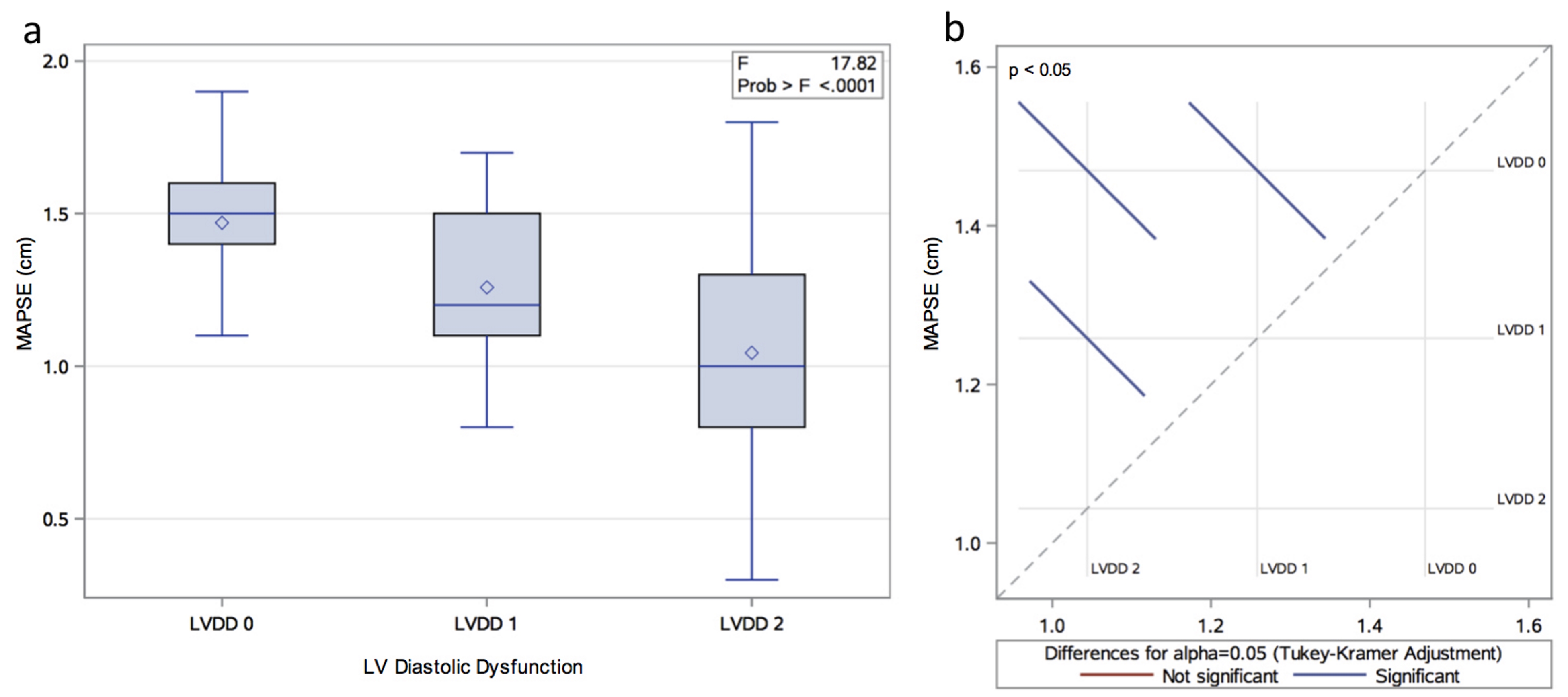 Click for large image | Figure 1. (a) Distribution of MAPSE by LVDD group. (b) Least square means adjustment for multiple comparisons using Bonferroni correction. The graphic shows significant differences between all the possible group pairs (LVDD 0 vs. 1; LVDD 0 vs. 2; LVDD 1 vs. 2). |
Correlation analysis between study variables and MAPSE
Significant linear correlation was observed between MAPSE and most of evaluated variables. Interestingly, age, LVDD, LA volume index (LAVI), LV mass index (LVMI), mitral velocity (MV) E/A and MV E/MA TDI E’ ratios were inversely correlated with MAPSE (P < 0.05). All the remaining variables showed a positive linear correlation (P < 0.05) with the exception of gender, BSA, MV E and MA TDI E’/A’ (Table 2). When groups of LVDD stages 1 and 2 were separately correlated with MAPSE using as a reference LVDD, we observed that the correlation coefficient significantly decreased as the LVDD worsened (-0.2114 and -0.4257, P < 0.01, respectively).
 Click to view | Table 2. Simple Linear Regression Analysis Between Evaluated Variables and MAPSE |
Development of a MAPSE predictive model and a two-way contour plot
All the relevant clinical and echocardiographic variables associated with MAPSE were evaluated by a stepwise selection method. As shown in Table 3, an initial multiple linear regression analysis was performed with age, LVEF, LAVI, LVMI and MV E/MA TDI E’. LVDD was included as a dummy variable, being the reference value LVDD 0, and both LVDD 1 and 2 were considered together as one category LVDD. Only age, LVEF and LVMI were significantly associated with MAPSE after adjusting for all evaluated variables. Hence, a nested model was constructed with age and LVEF and the final equation is represented as follow: MAPSE = 0.9063 - 0.0076*Age + 0.0113*LVEF.
 Click to view | Table 3. Stepwise Multiple Regression Analysis to Determine the Best Predictors of MAPSE |
Overall, 46% of the MAPSE variation was explained by this model (R2 = 0.4596, P < 0.01). A model diagnostics analysis shows a normal distribution of residuals and a good direct visual correlation between the observed versus fitted MAPSE values (Fig. 2). However, to ease the interpretation of the final model, a two-way contour illustrative graphic is depicted in Figure 3. Notice that a 60-year-old individual with a LVEF of 60% should have a MAPSE close to 1.2 cm, while in the case of an 80-year-old person with a LVEF of 30%, the MAPSE value should fall between 0.6 - 0.8 cm.
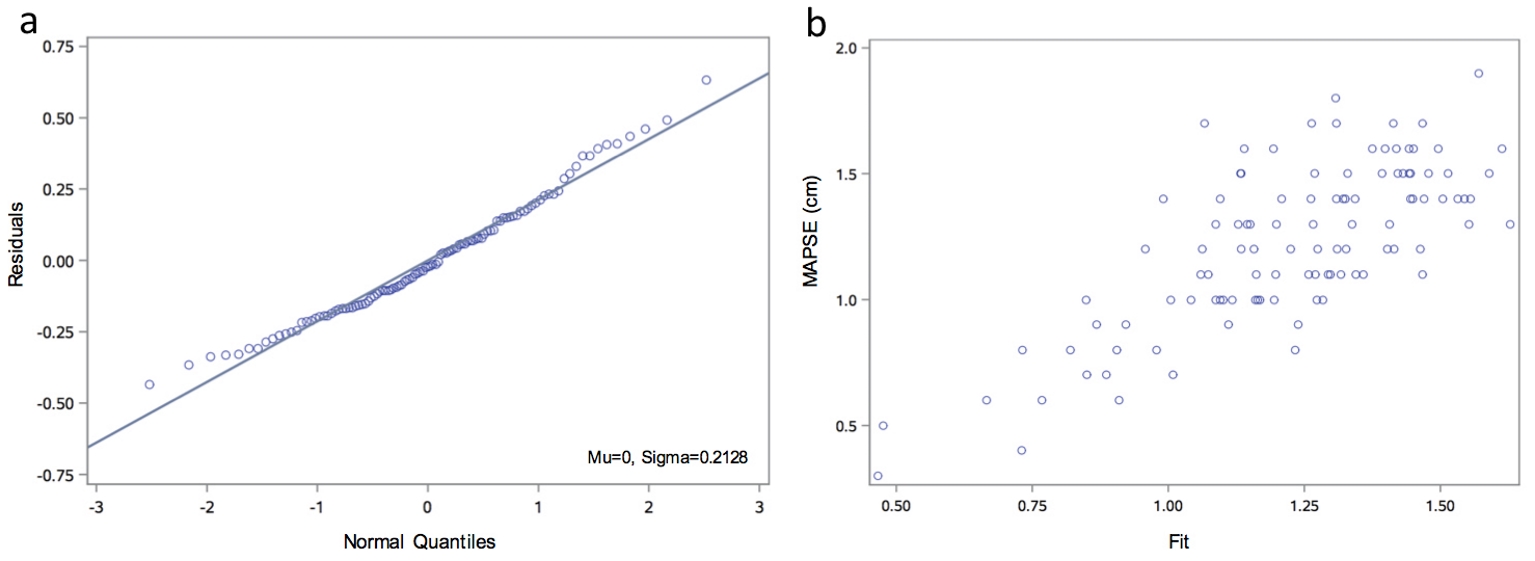 Click for large image | Figure 2. Model diagnostics analysis. (a) Q-Q plot for normality analysis of residuals showing a normal distribution. (b) Observed versus fitted MAPSE showing a good visual correlation. Q-Q: quantile-quantile. |
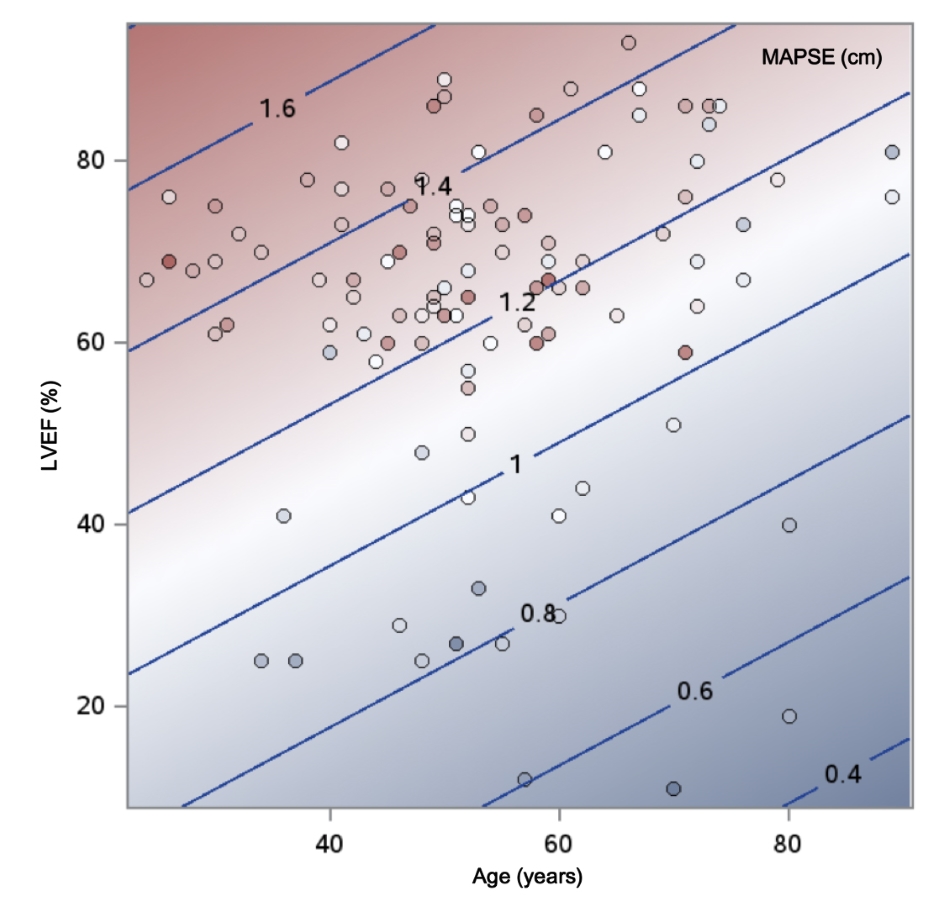 Click for large image | Figure 3. Contour fit plot for MAPSE by age and left ventricular ejection fraction. |
| Discussion | ▴Top |
Based on our results, the process of normal aging as well as increases in LV mass both diminish the longitudinal function of the LV reflected by a decreased MAPSE. Similarly, LVEF and mitral valve (MV) deceleration were positively associated with MAPSE. However, left atrial volume and pressure (as estimated by MV E / MA TDI E’ ratio) parameters were not correlated with MAPSE after adjusting for possible confounders, suggesting minimal contribution of the left atrial volumetric and pressure parameters to MA descent.
Several studies have shown a tendency to have a decreased MAPSE in hypertensive patients with normal systolic function [12, 13]. It is theorized that changes in subendocardial longitudinal fibers lead to a decreased base-to-apex contractility, which is compensated in the early stages by augmented radial function, thereby preserving EF.
MA motion has been also associated with abnormal LV diastolic filling pressures and elevated LV mass [14]. Some studies have also revealed that brain natriuretic peptide (BNP) levels correlate with worsening MAPSE [15]. It is possible that high filling pressures generated by the LA restrict the net ascent of the MA, therefore diminishing its net descent. MAPSE may therefore be a sensitive marker of global LV function.
Moreover, we proposed a simplified model to predict MAPSE by multiple regression analysis. To our knowledge, this is the first study in which an algorithm to predict MA motion in LVDD patients has been developed. Additionally, a graphical representation was illustrated to ease the model interpretation. However, whether this algorithm may have clinical relevance needs to be further tested.
Our study has some limitations. First, it is of retrospective nature and has a small simple size. Second, our results may not be generalizable to patients with arrhythmias and/or significant valvular disease, as they were excluded. Third, we had no speckle tracking imaging information; consequently, information that could improve the impact of MAPSE in LVDD was not included. However, we show preliminary results in the assessment of MA motion in diastolic dysfunction that might set a benchmark to further speckle tracking studies. Likewise, it would be interested to have included cine cardiovascular magnetic resonance data considering recent report of Rangarajan et al and associates on the impact of reduced long axis function over major adverse cardiovascular events [6]. Finally, although recommendations for the evaluation of LV diastolic function by echocardiography have been recently updated [16], yet most of LVDD patients remain under this classification by new guidelines. Thus, since echocardiographic measurement of MAPSE remains unchanged, we anticipate that our results would not have significantly differed if recent LVDD classification criteria are used.
Conclusions
In summary, our study highlights the importance of age and LV measures in determining MAPSE in patients with diastolic dysfunction. Furthermore, a simplified model to predict MAPSE based on age and LVEF with an appropriate illustrative representation to ease its interpretation is proposed. Additional studies are now warranted to explore whether a reduced MAPSE may affect the symptomatic profile or the overall prognosis in LVDD patients.
Acknowledgments
None to declare.
Funding
This publication was partially supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health Award Numbers U54MD007587, U54MD007600, S21MD001830, and CCTRECD-R25MD007607. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
None to declare.
| References | ▴Top |
- Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, Ertl G, et al. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14(3):205-212.
doi pubmed - Rydberg E, Arlbrandt M, Gudmundsson P, Erhardt L, Willenheimer R. Left atrioventricular plane displacement predicts cardiac mortality in patients with chronic atrial fibrillation. Int J Cardiol. 2003;91(1):1-7.
doi - Brand B, Rydberg E, Ericsson G, Gudmundsson P, Willenheimer R. Prognostication and risk stratification by assessment of left atrioventricular plane displacement in patients with myocardial infarction. Int J Cardiol. 2002;83(1):35-41.
doi - Svealv BG, Olofsson EL, Andersson B. Ventricular long-axis function is of major importance for long-term survival in patients with heart failure. Heart. 2008;94(3):284-289.
doi pubmed - Wenzelburger FW, Tan YT, Choudhary FJ, Lee ES, Leyva F, Sanderson JE. Mitral annular plane systolic excursion on exercise: a simple diagnostic tool for heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(9):953-960.
doi pubmed - Rangarajan V, Chacko SJ, Romano S, Jue J, Jariwala N, Chung J, Farzaneh-Far A. Left ventricular long axis function assessed during cine-cardiovascular magnetic resonance is an independent predictor of adverse cardiac events. J Cardiovasc Magn Reson. 2016;18(1):35.
doi pubmed - Hernandez Burgos PM, Lopez-Candales A. Changes in Mitral Annular Ascent with Worsening Echocardiographic Parameters of Left Ventricular Diastolic Function. Scientifica (Cairo). 2016;2016:6303815.
doi - Tasolar H, Mete T, Cetin M, Altun B, Balli M, Bayramoglu A, Otlu YO. Mitral annular plane systolic excursion in the assessment of left ventricular diastolic dysfunction in obese adults. Anatol J Cardiol. 2015;15(7):558-564.
doi pubmed - Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107-133.
doi pubmed - Lopez-Candales A, Rajagopalan N, Gulyasy B, Edelman K, Bazaz R. Comparative echocardiographic analysis of mitral and tricuspid annular motion: differences explained with proposed anatomic-structural correlates. Echocardiography. 2007;24(4):353-359.
doi pubmed - Hernandez-Suarez DF, Lopez Menendez FR, Palm D, Lopez-Candales A. Left ventricular diastolic function assessment of a heterogeneous cohort of pulmonary arterial hypertension patients. J Clin Med Res. 2017;9(4):353-359.
doi pubmed - Ballo P, Quatrini I, Giacomin E, Motto A, Mondillo S. Circumferential versus longitudinal systolic function in patients with hypertension: a nonlinear relation. J Am Soc Echocardiogr. 2007;20(3):298-306.
doi pubmed - Xiao HB, Kaleem S, McCarthy C, Rosen SD. Abnormal regional left ventricular mechanics in treated hypertensive patients with 'normal left ventricular function'. Int J Cardiol. 2006;112(3):316-321.
doi pubmed - Willenheimer R, Israelsson B, Cline C, Rydberg E, Broms K, Erhardt L. Left atrioventricular plane displacement is related to both systolic and diastolic left ventricular performance in patients with chronic heart failure. Eur Heart J. 1999;20(8):612-618.
doi pubmed - Elnoamany MF, Abdelhameed AK. Mitral annular motion as a surrogate for left ventricular function: correlation with brain natriuretic peptide levels. Eur J Echocardiogr. 2006;7(3):187-198.
doi pubmed - Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


