| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 9, Number 6, December 2018, pages 370-377
Clinical Outcomes of World’s Thinnest (50 μmr) Strut Biodegradable Polymer Coated Everolimus-Eluting Coronary Stent System in Real-World Patients
Suresh V. Patteda, c, Anmol Suresh Patteda, Prakash Kumar Turiyab, Ashok S. Thakkarb, c
aKLE Academy of Higher Education and Research Centre, KLE University, Belgaum, Karnataka, 590010, India
bMeril Life Sciences Pvt. Ltd., Vapi, Gujarat, 396191, India
cCorresponding Author: Suresh V. Patted, Department of Cardiology, KLE Academy of Higher Education and Research, KLE University, Belgaum 590010, Karnataka, India; Ashok S. Thakkar, Clinical Research and Medical Writing, Meril Life Sciences Pvt. Ltd., Vapi, Gujarat, 396191, India
Manuscript submitted October 26, 2018, accepted November 12, 2018
Short title: The Evermine 50 EES – 1 BGM study
doi: https://doi.org/10.14740/cr800
| Abstract | ▴Top |
Background: The thinnest strut platform is revolutionary improvement into the field of percutaneous coronary intervention. The aim of this study was to assess the safety and performance of world’s thinnest (50 µm) strut biodegradable polymer coated Evermine 50™ everolimus-eluting coronary stent system (EES) in real-world patients with coronary artery disease.
Methods: This was a prospective, single-arm, single-center, post-marketing study in real-world patients. A total of 251 patients with de novo coronary artery lesion (lengths < 44 mm) and/or in-stent restenosis were enrolled and implanted with at least one Evermine 50 EES. The safety endpoint was major adverse cardiac events (MACE), composite of cardiac death, myocardial infarction (MI) attributed to the target vessel and clinically-driven target lesion revascularization (CD-TLR), at 6-month follow-up.
Results: Out of 251 patients enrolled (mean age: 58.20 ± 9.92 years and 193 males), 48.6% and 45.4% patients were diabetic and hypertensive, respectively. A total of 343 lesions were intervened successfully with Evermine 50 out of 474 identified lesions (1.89 lesions per patients). Average stent length and diameter were 23.50 ± 12.21 mm and 2.83 ± 0.23 mm, respectively. At 6-month follow-up, the incidence of MACE was two (0.8%) in the form of one (0.4%) cardiac death and one (0.4%) CD-TLR. In addition, there was no definite or probable stent thrombosis reported up to 6-month follow-up.
Conclusions: In the present study, lower rate of MACE was demonstrated, which reaffirms favourable clinical safety and performance of world’s thinnest (50 µm) strut Evermine 50 EES in real-world patients with coronary artery disease.
Keywords: Biocompatible; Biodegradable; De novo coronary lesions; Everolimus-eluting coronary stent system; Restenotic lesions; Thinnest strut
| Introduction | ▴Top |
Percutaneous coronary intervention (PCI) has revolutionized the treatment of patients with coronary artery disease (CAD). However, the first-generation drug-eluting stent (DES) was linked with comparatively high revascularization rates and risk of late events including stent thrombosis (ST), which enlightened the opportunity for further improvement in DES technology [1]. This lead to introduction of second-generation DES which have markedly improved clinical outcomes in patients undergoing PCI by reducing the risk of restenosis, myocardial infarction (MI), and improve survival as compared with bare metal stent (BMS) and first-generation DES [2, 3]. Moreover, second-generation DES demonstrated lower rate of thrombotic occlusion after stent implantation with no major difference among cobalt-chromium (Co-Cr) EES, Co-Cr zotarolimus-eluting stent or platinum-chromium EES in large randomized controlled trials [4-8].
A previous study suggested that the response with biodegradable polymer coated EES was favourable in terms of healing, neointimal growth suppression, and inflammation as compared to durable polymer DES and BMS [9]. In addition, DES with thinner struts reduces cardiovascular injury and inflammation, and promotes faster endothelialization, decreasing thrombogenicity and neointimal proliferation [10]. Furthermore, revealed from the several studies, an ultrathin strut (Orsiro (60 µm), MiStent (64 µm), BioMime (65 µm), SYNERGY (74 µm)) was the most commonly used stent for the treatment of coronary artery lesions [11-14].
The Evermine 50 (Meril Life Sciences Pvt. Ltd., India) is the thinnest (50 µm) strut biodegradable polymer coated EES system which has been developed on the Co-Cr platform. The thinnest strut platform has recently been commercialised and is emerging tool for the treatment of de novo lesions and/or in-stent restenosis for CAD patients. The aim of the present study was to evaluate the safety and performance of Evermine 50 EES in the treatment of real-world CAD patients.
| Materials and Methods | ▴Top |
Study design and population
This was a prospective, single-arm, single-center, post-marketing, real-world study conducted at tertiary care center, India (CTRI number: CTRI/2017/03/008173). Patients aged above 18 years with stable CAD or acute coronary syndrome, having de novo native coronary artery lesions (lengths < 44 mm) and/or in-stent restenosis with a reference vessel diameter of 2.0 mm to 4.5 mm, and compatible to Evermine 50 EES implantation were eligible for study. Patients with known allergies to aspirin, heparin, everolimus, polymer lactide, Co-Cr metal and glycolide antiplatelet drugs (clopidogrel, prasugrel etc.) and/or those who had already participated in another trial before reaching primary endpoint of the present study, were excluded from the study. The study was approved by institutional ethics committee, and was conducted according to Declaration of Helsinki, local regulations and ISO 14155. Written informed consents were obtained from all the study patients prior to study procedure.
All the patients were administered with a loading dose of aspirin (150 - 325 mg) and ticagrelor (180 mg) or clopidogrel (75 - 600 mg) before procedure. Intravenous heparin (70 - 100 units/kg) was administered to preserve an activated clotting time > 250 s. The PCI was performed according to standard guidelines. All patients received post-procedural dual anti-platelet therapy with aspirin (75 - 150 mg/day) and clopidogrel (75 mg/day) or prasugrel (10 mg/day) or ticagrelor (180 mg/day) for 1 year. After 1 year, patients were suggested to mono anti-platelet therapy as per ACC/AHA guideline [15].
Device description
The Evermine 50 EES (Meril Life Sciences Pvt. Ltd., India) is built on thinnest (50 µm) strut Co-Cr platform, and is coated with biodegradable polymers: PLGA (poly-lactic-co-glycolic acid) and PLLA (poly-L-lactic acid) as shown in Figure 1. The device has been approved by Conformite Europeene (CE) mark. Evermine 50 EES uses unique hybrid cell design comprising of an intelligent mix of open cells in the mid segment and closed cells at the edges. The Evermine 50 EES elutes everolimus (1.25 µg/mm2 of stent area) as an anti-proliferative drug. The Evermine 50 EES is available in lengths of 8, 13, 16, 19, 24, 29, 32, 37, 40, 44, and 48 mm and diameters of 2.00, 2.25, 2.50, 2.75, 3.00, 3.50, 4.00, and 4.50 mm.
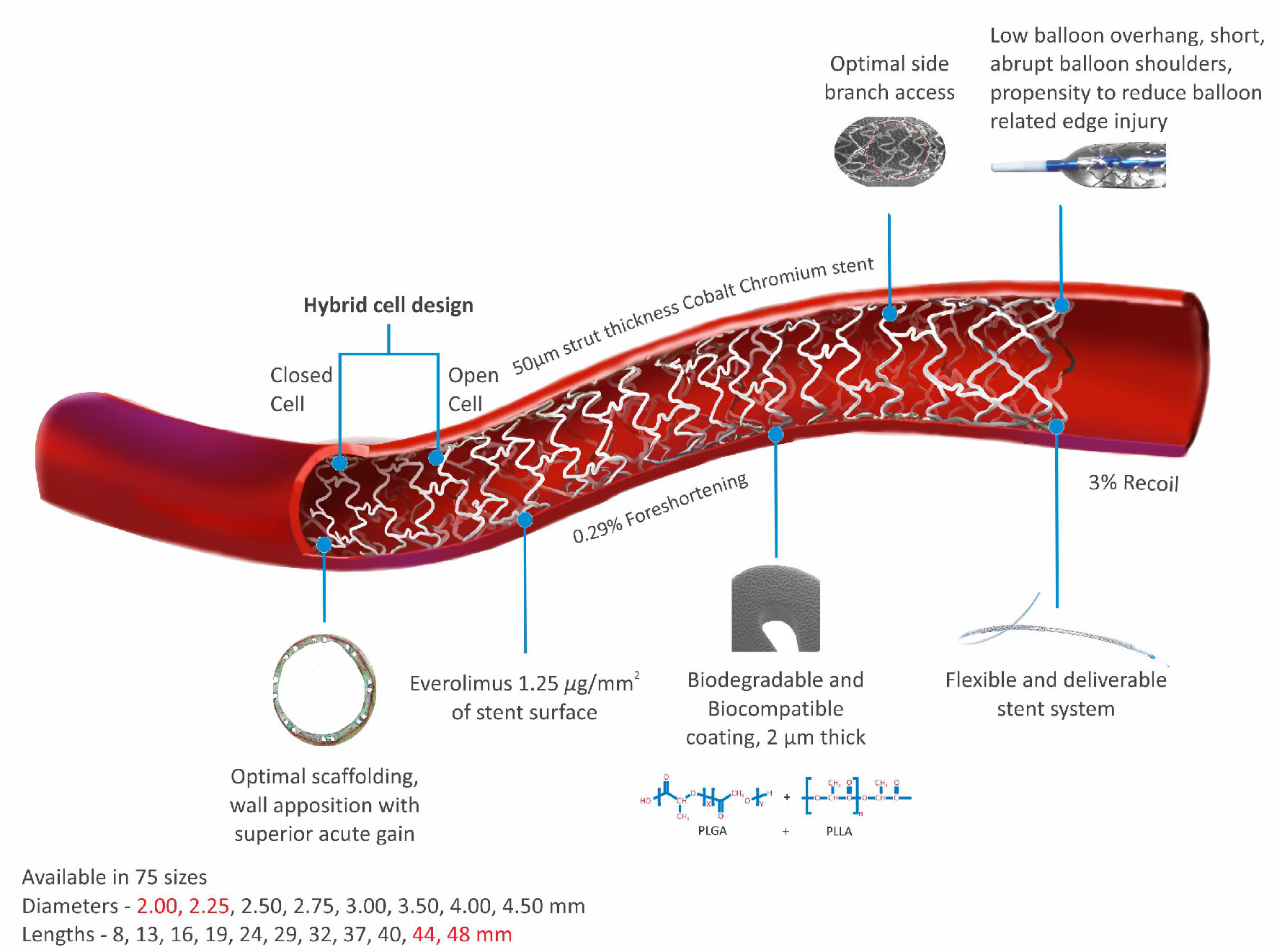 Click for large image | Figure 1. Evermine 50 EES thinnest (50 µm) strut platform. |
Concept of thinner strut platform
Conceptually, the thinnest struts provide low blood flow perturbance and easy strut nesting to the vessel wall and also additional flexibility and conformability during the process of implantation [16]. Flow dynamics based on strut thickness is shown in Figure 2. The presence of thicker strut shows high protrusion into the lumen which alters blood vessel flow dynamics, creating areas of turbulent flow with shear stress. These areas have been allied to activation of platelets and upregulation of smooth muscle cell proliferation, which may additionally worsen the pathology of an already injured vessel [17, 18]. In contrast, a laminar flow model linked to poorer blood flow disturbance is often seen as preferable as it mimics physiological setting [19]. Hence, more supportive in reducing the formation of in-stent restenosis and maintained the endothelialization capacity with lower strut thickness up to 75 µm [20, 21].
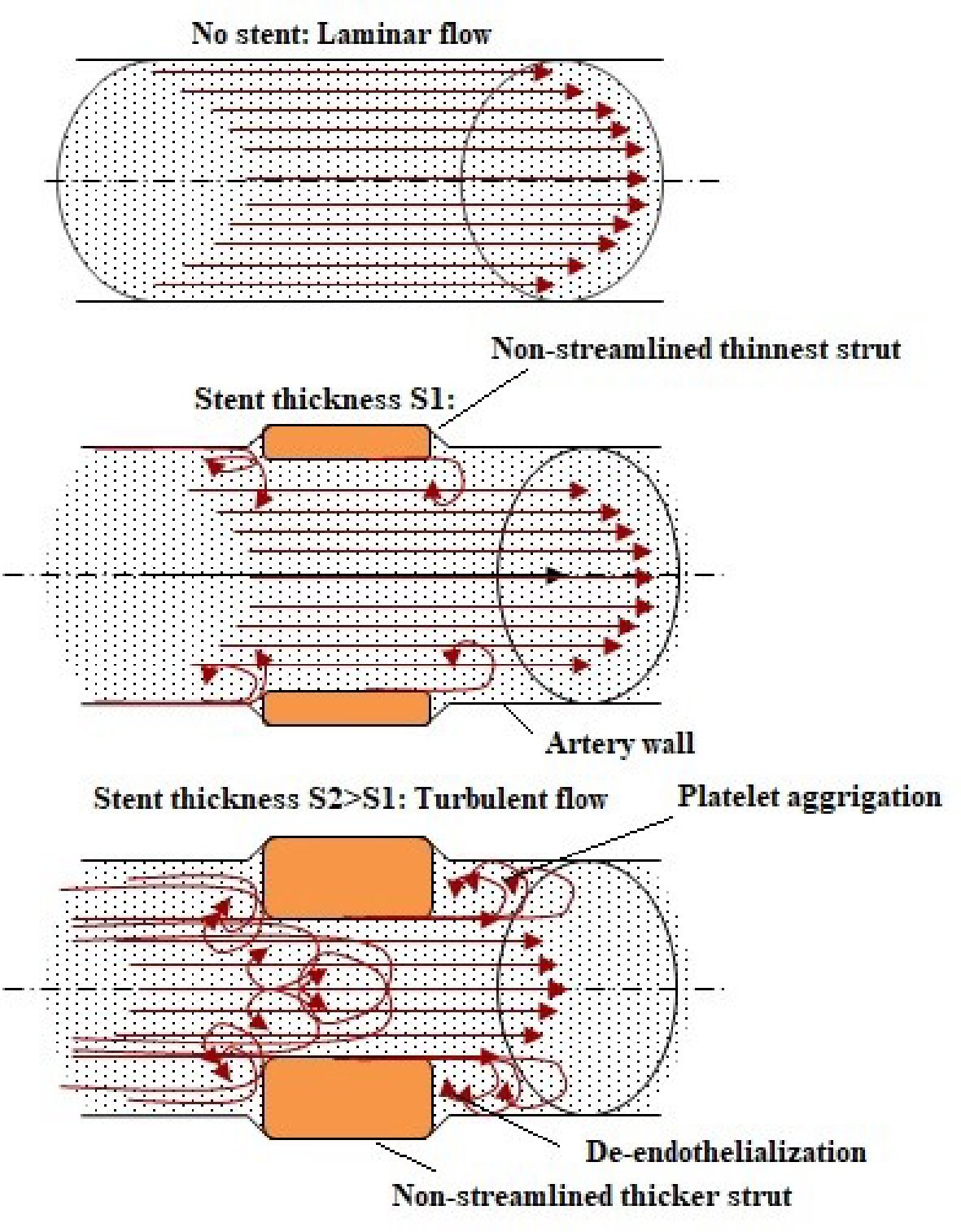 Click for large image | Figure 2. Flow dynamics based on strut thickness. |
Definitions and endpoints
The safety endpoint was the occurrence of major adverse cardiac events (MACE), composite of cardiac death, myocardial infarction (MI) attributed to the target vessel, and clinically-driven target lesion revascularization (CD-TLR) at 6-month follow-up after the index procedure. Cardiac death was defined as any death due to acute MI, stroke or heart failure, death related to procedure, or unknown cause. MI was defined as development of new pathological Q waves on electrocardiogram, or elevation of creatinine kinase (CK) ≥ 2 fold the upper limit of normal with elevated CK-MB in the absence of new pathological Q waves or new ischemic symptoms [22]. CD-TLR was defined as repeat PCI or revascularisation as clinically indicated or coronary artery bypass graft surgery triggered by clinically indicated repeat coronary angiography. Stent thrombosis (ST) was classified according to the definitions of the Academic Research Consortium [23]. Procedural success was defined as successful stent placement at the desired position without any death, MI, or repeat revascularization of target lesion during the hospital stay. Device success was defined as achievement of a final residual diameter stenosis < 30% by visual estimation using study device only. All adverse events were adjudicated by an independent clinical event committee.
Statistical analysis
The demographic and baseline characteristics were summarized using the descriptive statistics. For continuous variable such as age, data were presented as mean ± SD. The categorical variables such as gender, risk factors, cardiac status were presented as frequency and percentages. Percentage was calculated according to the number of patients for whom data were available. All the statistical analysis was performed using software SPSS version 15 (SPSS Inc, Chicago, IL, USA). The event-free survival rate was analyzed by the Kaplan-Meier method.
| Results | ▴Top |
Demographic data and baseline characteristics
A total of 251 patients, based on inclusion and exclusion criteria, were enrolled from March 2017 to April 2018. All the enrolled patients had at least one de novo coronary artery lesion and successfully underwent PCI with at least one Evermine 50 EES for the treatment. Out of 251 patients (mean age: 58.20 ± 9.92 years), 193 (76.9%) were male. A total of 122 (48.6%) patients had diabetes mellitus, 29 (11.6%) patients consumed alcohol, and 114 (45.4%) patients were hypertensive. Approximately half of patients (49.4%) had single vessel disease, followed by 35.1% and 15.5% patients with double and triple vessel disease, respectively. Baseline demographics and clinical characteristics of study population are shown in Table 1.
 Click to view | Table 1. Baseline Demographic and Clinical Characteristics |
Procedural and lesion characteristics
A total of 474 lesions were identified in 251 patients (1.89 lesions per patient), out of which 343 lesions with different type of stenosis (337 (98.2%) de novo, three (0.9%) in-stent, and three (0.9%) bifurcations) were intervened successfully with Evermine 50 EES (1.37 stent per patient). The most common target vessel treated was left anterior descending artery in 173 patients (50.4%); followed by right coronary artery (31.2%), left circumflex (16.9%) and ramus (1.5%). Pre-dilatation was performed in 76.4% of the lesions while post-dilatation was carried out in 57.1% of the lesions. The average diameter and length of implanted study stent was 2.83 ± 0.23 mm and 23.50 ± 12.21 mm, respectively. Approximately half of the lesions treated with Evermine 50 were type B1 lesions 160 (46.6%), and type A, type B2 and type C lesions were 108 (31.5%), 21 (6.1%) and 54 (15.7%), respectively. In addition there were 100% procedure and device success achieved without any adverse event during index procedure. The lesion characteristics of patients are given in Table 2. Additionally, procedural characteristics and medication at discharge is mentioned in Table 3.
 Click to view | Table 2. Lesions Characteristics |
 Click to view | Table 3. Procedural Characteristics |
Clinical outcomes
The follow-up at 6-month was completed in 100% patients. MACE occurred in two (0.8%) patients in the form of one (0.4%) cardiac death due to sudden cardiac arrest and one (0.4%) CD-TLR. None of the patient experienced MI or ST at 6-month follow-up. A single (0.4%) case of non-cardiac death was caused due to pneumonia. The summary of cumulative MACE data is shown in Table 4. The time-to-event curve by Kaplan-Meier method at 6-month follow-up is shown in Figure 3.
 Click to view | Table 4. Cumulative Clinical Outcomes at 6-Month Follow-up |
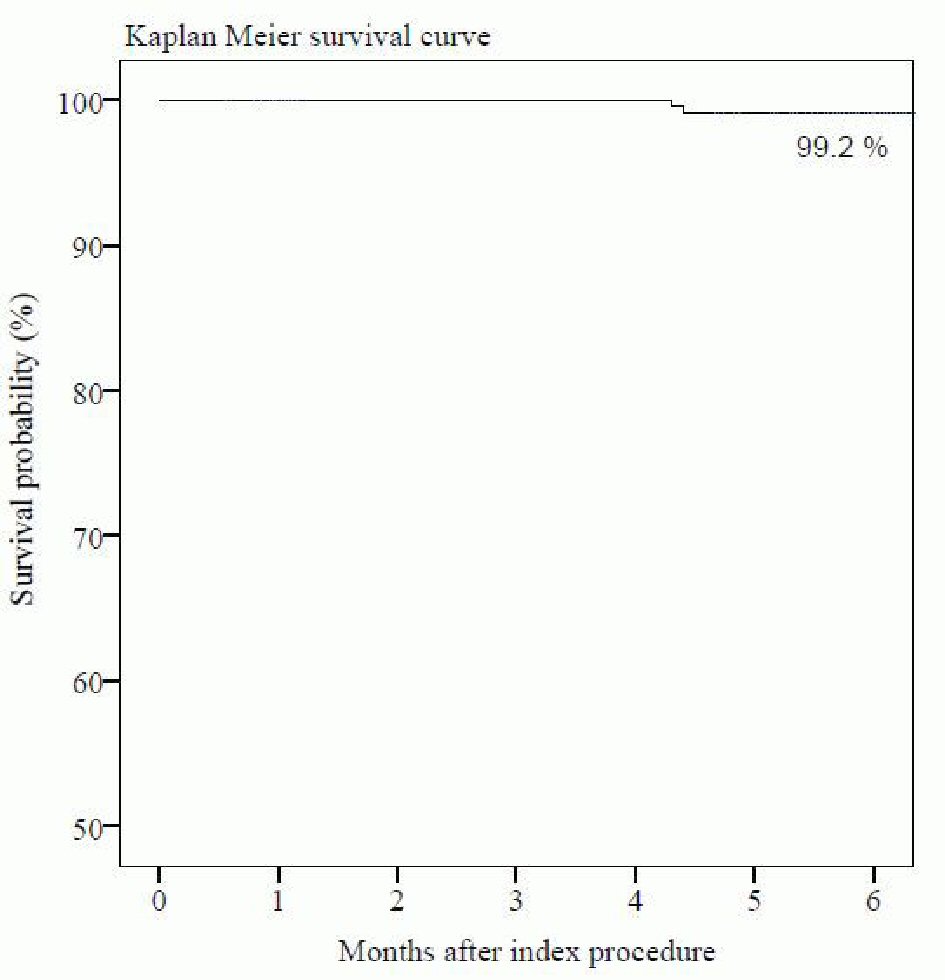 Click for large image | Figure 3. Time-to-event curve by Kaplan-Meier method at 6-month follow-up. |
Amongst the two patients who had cardiac and non-cardiac death, the cardiac death patient was a 64-year-old male with history of angina and triple vessel disease and underwent PCI with the study device. Furthermore, he was discharged at post percutaneous transluminal coronary angioplasty in stable condition. At 1 month follow-up, patient complained of pain at upper left side of chest and investigator prescribed him medication. His death was reported within 6 months after PCI due to sudden cardiac arrest. The non-cardiac death occurred in a 75-year-old male patient due to pneumonia at 6-month follow-up.
| Discussion | ▴Top |
In this study we assessed the clinical safety and performance of world’s thinnest strut platform in real-world patients. The present study reported two (0.8%) MACE, which includes one (0.4%) cardiac death and one (0.4%) CD-TLR. There was absence of MI and ST at 6-month follow-up. Additionally, 100% procedural and device success rates were achieved. Diabetes mellitus and hypertension are well-known risk factors for CAD and in the present study, there was high occurrence of diabetes mellitus (48.6%) and hypertension (45.4%). Evermine 50 EES is designed with thinnest (50 µm) strut that allows low blood flow perturbance, fast arterial healing, faster endothelialization and reduction of in-stent restenosis [24].
In recent published editorial, Evermine 50 EES reported 1.8% MACE in 171 patients at 12-month follow-up of ongoing study with no event of ST [25]. Moreover, in other studies with large number of patients, cobalt-chromium EES (XIENCE V or PROMUS) is associated with a lower rate of MACE and ST at long-term follow-up period [26]. Recently, a meta-analysis of randomized trial demonstrated that newer generation ultrathin strut DES in patients undergoing PCI improved clinical outcomes at 1-year follow-up compared with thicker strut second generation DES [27]. Previously published (ISAR-STEREO) trial represented randomized clinical trial of thin-strut (50 µm) ACS RX Multilink stent vs. thick-strut (140 µm) ACS Multi-Link RX Duet. This trial demonstrated the beneficial role of thin-strut in re-endothelialization in CAD after stenting. There was less angiographic restenosis in the thin-strut group (15.0%) vs. thick-strut group (25.8%) with relative risk (0.58; 95% CI: 0.39 - 0.87; P = 0.003) [21].
Moreover, some clinical trials have identified stent strut thickness as an independent predictor of stent restenosis [21, 28-30]. Previous published study demonstrated that the implantation of coronary stents constructed with thin metal struts is associated with a significant reduction of clinical (-38%) and angiographic (-42%) restenosis when compared with a stent having strut thickness twice as great [21]. The present study reported lower rate of MACE in real-world patients with CAD. In a randomized control trial by Sabate et al, EES group reported significant reduction in MI related to target vessel (-0.94 (-1.19 to 0.30), P = 0.14) and TLR (-2.82 (-4.69 to 0.96), P = 0.0032) when compared with bare metal stent group at 1-year follow-up. In addition, the ST in BMS group was almost 3 fold higher than EES group [31]. In a study by Kitabata et al, the MACE rate was lower in thin-strut Xience V EES (1.8%) when compared with Cypher sirolimus-eluting stents (4.9%) and Taxus paclitaxel-eluting stents (5.1%), at 30 days [32].
Moreover, revolutionary improvement in DES technology with ultrathin strut provides better option instead of thicker strut as depicted from previous ultrathin EES studies. The stability of EES has been demonstrated by the above published studies in comparison with implanted BMS, sirolimus-eluting stents and paclitaxel-eluting stents in patients who have CAD or complex type of lesions. The present study demonstrated favourable clinical outcomes with the use of world’s thinnest (50 µm) strut Evermine 50 EES in real-world patients with CAD.
The present study has several potential limitations. Starting with, sample size considered for the study is small. Moreover, this was a non-randomized and single-arm study. This study lacks angiographic data and hence, we could not detect any possible complications like stent fracture at the deployment site nor the angiographic characteristics of restenosis with ultrathin strut.
Conclusions
The outcomes of the study demonstrated lower incidence of the MACE after implantation of biodegradable polymer coated thinnest (50 µm) strut Evermine 50 EES, which depicts favourable clinical safety and performance in patients with coronary artery disease. These results provide the base for future randomized trials to test whether Evermine 50 EES could be comparable to the other contemporary DES for clinical outcomes.
Acknowledgments
The authors would like to thank Vipin Bulani, PhD, Nadeem Sayyed, M. Pharm, Priyanka J. Desai, M. Pharm and Prem Sahu, M.Sc. for their assistance in the preparation of manuscript. Also, thanks to all the included patients in this study.
Funding
This study was partially funded by Meril Life Sciences Pvt. Ltd.
Conflict of Interest
Dr. Ashok S. Thakkar and Prakash Kumar Turiya are full-time employees of Meril Life Science, Pvt. Ltd., India. The other authors have no potential conflict of interest to declare.
Disclosure
None.
| References | ▴Top |
- Panoulas VF, Mastoris I, Konstantinou K, Tespili M, Ielasi A. Everolimus-eluting stent platforms in percutaneous coronary intervention: comparative effectiveness and outcomes. Med Devices (Auckl). 2015;8:317-329.
- Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, Wenaweser P, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125(9):1110-1121.
doi pubmed - Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125(23):2873-2891.
doi pubmed - Toyota T, Shiomi H, Morimoto T, Kimura T. Meta-analysis of long-term clinical outcomes of everolimus-eluting stents. Am J Cardiol. 2015;116(2):187-194.
doi pubmed - Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, Vlachojannis GJ, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol. 2015;65(23):2496-2507.
doi pubmed - Bonaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygard O, Nilsen DW, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375(13):1242-1252.
doi pubmed - Jensen LO, Thayssen P, Christiansen EH, Maeng M, Ravkilde J, Hansen KN, Hansen HS, et al. Safety and Efficacy of Everolimus- Versus Sirolimus-Eluting Stents: 5-Year Results From SORT OUT IV. J Am Coll Cardiol. 2016;67(7):751-762.
doi pubmed - Piccolo R, Stefanini GG, Franzone A, Spitzer E, Blochlinger S, Heg D, Juni P, et al. Safety and efficacy of resolute zotarolimus-eluting stents compared with everolimus-eluting stents: a meta-analysis. Circ Cardiovasc Interv. 2015;8(4):e002223.
doi pubmed - Mori H, Atmakuri DR, Torii S, Braumann R, Smith S, Jinnouchi H, Gupta A, et al. Very Late Pathological Responses to Cobalt-Chromium Everolimus-Eluting, Stainless Steel Sirolimus-Eluting, and Cobalt-Chromium Bare Metal Stents in Humans. J Am Heart Assoc. 2017;6(11):e007244.
doi pubmed - Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123(13):1400-1409.
doi pubmed - Iglesias JF, Roffi M, Degrauwe S, Secco GG, Aminian A, Windecker S, Pilgrim T. Orsiro cobalt-chromium sirolimus-eluting stent: present and future perspectives. Expert Rev Med Devices. 2017;14(10):773-788.
doi pubmed - Shreenivas SS, Kereiakes DJ. Evolution of the SYNERGY bioresorbable polymer metallic coronary stent. Future Cardiol. 2018;14(4):307-317.
doi pubmed - Abizaid A, Kedev S, Kedhi E, Talwar S, Erglis A, Hlinomaz O, Masotti M, et al. Randomized Comparison of Biodegradable Polymer Ultra-thin Sirolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Coronary Stent in Patients with De Novo Native Coronary Artery Lesions: The meriT-V Trial. Euro Intervention. 2018.
- Wijns W, Vrolix M, Verheye S, Schoors D, Slagboom T, Gosselink M, Benit E, et al. Long-term clinical outcomes of a crystalline sirolimus-eluting coronary stent with a fully bioabsorbable polymer coating: five-year outcomes from the DESSOLVE I and II trials. EuroIntervention. 2018;13(18):e2147-e2151.
doi pubmed - Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123-155.
doi pubmed - Farhatnia Y, Pang JH, Darbyshire A, Dee R, Tan A, Seifalian AM. Next generation covered stents made from nanocomposite materials: A complete assessment of uniformity, integrity and biomechanical properties. Nanomedicine. 2016;12(1):1-12.
doi pubmed - Duraiswamy N, Schoephoerster RT, Moreno MR, James E, Moore J. Stented artery flow patterns and their effects on the artery wall. Annual Review of Fluid Mechanics. 2007;39(1):357-382.
doi - Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327-387.
doi pubmed - Jimenez JM, Davies PF. Hemodynamically driven stent strut design. Ann Biomed Eng. 2009;37(8):1483-1494.
doi pubmed - Kereiakes DJ, Cox DA, Hermiller JB, Midei MG, Bachinsky WB, Nukta ED, Leon MB, et al. Usefulness of a cobalt chromium coronary stent alloy. Am J Cardiol. 2003;92(4):463-466.
doi - Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, Fleckenstein M, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103(23):2816-2821.
doi pubmed - Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mahonen M, Ngu Blackett K, Lisheng L, et al. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40(1):139-146.
doi pubmed - Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351.
doi pubmed - Milewski K, Gasior P, Samborski S, Buszman PP, Blachut A, Wojtaszczyk A, Mlodziankowski A, et al. Evaluation of safety and efficacy of NexGen - an ultrathin strut and hybrid cell design cobalt-chromium bare metal stent implanted in a real life patient population - the Polish NexGen Registry. Postepy Kardiol Interwencyjnej. 2016;12(3):217-223.
doi - Patted SV. Biodegradable polymer Evermine 50™ everolimus eluting coronary stent system with ultrathin (50 µm) strut. Int Clin Med. In Press 2018.
doi - Aoki J, Kozuma K, Awata M, Nanasato M, Shiode N, Tanabe K, Yamaguchi J, et al. Three-year clinical outcomes of everolimus-eluting stents from the post-marketing surveillance study of Cobalt-Chromium Everolimus-Eluting Stent (XIENCE V/PROMUS) in Japan. Circ J. 2016;80(4):906-912.
doi pubmed - Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer Generation Ultra-Thin Strut Drug-Eluting Stents versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease: A Meta-Analysis of Randomized Trials. Circulation. 2018.
doi - Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, Sgura F, et al. In-stent restenosis in small coronary arteries: impact of strut thickness. J Am Coll Cardiol. 2002;40(3):403-409.
doi - Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, Fleckenstein M, et al. [Intracoronary Stenting and Angiographic Results Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO) Trial]. Vestn Rentgenol Radiol. 2012;2:52-60.
- Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41(8):1283-1288.
doi - Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380(9852):1482-1490.
doi - Kitabata H, Loh JP, Pendyala LK, Badr S, Dvir D, Barbash IM, Minha S, et al. Safety and efficacy outcomes of overlapping second-generation everolimus-eluting stents versus first-generation drug-eluting stents. Am J Cardiol. 2013;112(8):1093-1098.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


