| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Case Report
Volume 9, Number 6, December 2018, pages 392-394
Thrombotic Thrombocytopenic Purpura Following Aortic Valve Replacement with St. Jude Medical Trifecta Bio-Prosthesis
Andrea D’Alessioa, Danilo Verdichizzoa, Fabio Falconieria, Amar Keirallaa, Joanna Abramikb, George Kassimisb, c, George Krasopoulosa, d, e
aOxford Heart Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
bCardiology Department, Gloucestershire Hospitals NHS Foundation Trust, Cheltenham General Hospital, Cheltenham, UK
cSecond Department of Cardiology, Hippokration Hospital, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
dUniversity of Oxford, Oxford, UK
eCorresponding Author: George Krasopoulos, Oxford Heart Centre, Oxford University Hospitals NHS Foundation Trust, Headley Way, Headington, Oxford OX3 9DU, UK
Manuscript submitted September 6, 2018, accepted September 27, 2018
Short title: TTP After AVR With Trifecta
doi: https://doi.org/10.14740/cr780w
| Abstract | ▴Top |
Thrombocytopenia is a recognized complication following aortic valve replacement (AVR). While post-operative thrombotic thrombocytopenic purpura (TTP) is less common than heparin-induced thrombocytopenia (HIT), it is associated with high mortality and morbidity and prompt diagnosis and treatment is vital. In this case report, we describe the first reported case of TTP after AVR using the trifecta bio-prosthesis. We recommend that patients with severe and progressive thrombocytopenia following biological AVR should have early screening for both HIT and TTP, to shorten the decision-making process and provide the appropriate therapy.
Keywords: Heparin-induced thrombocytopenia; Thrombotic thrombocytopenic purpura; Aortic valve replacement; Bio-prosthetic valve
| Introduction | ▴Top |
Thrombocytopenia is a recognized complication following aortic valve replacement (AVR). It is associated with the type of valve prosthesis implanted, the duration of the extracorporeal circulation and anti-coagulation therapy received during the first post-operative hours [1]. While often multifactorial, it is commonly attributed to heparin use and is associated with high mortality and morbidity [2]. However, a much rarer complication, thrombotic thrombocytopenic purpura (TTP), is often under-recognized due to physicians’ lack of familiarity with the condition [3]. This case report describes, to the best of our knowledge, the first reported case of TTP after AVR with the use of trifecta (St. Jude Medical, Little Canada, MN, USA) bio-prosthetic valve.
| Case Report | ▴Top |
A 66-year-old female with symptomatic severe aortic stenosis and mildly impaired left ventricular systolic function was referred to our center for AVR. Her past medical history included one episode of transient ischemic attack and atrial fibrillation of recent onset; therefore, she was started on low molecular weight heparin (LMWH) for a period of 2 weeks prior to her surgery date.
Her preoperative hemoglobin (Hb) was 136 mg/dL and platelet count (PC) was 253 × 109/L. She underwent biological AVR with a 23 mm St. Jude Medical trifecta bio-prosthesis. The operation was uneventful and post-operative echocardiogram confirmed a well-functioning AVR prosthesis with no evidence of peri-prosthetic leak. We are usually perform routine blood tests (full blood count, prothrombin time (PT), activated partial thromboplastin time (APTT), electrolytes, urea, creatinine and liver function tests) at day 2 and 4 post-operatively. On the second post-operative day (POD2) the PC dropped (125 × 109/L), with a further reduction at POD4 to 52 × 109/L, despite discontinuation of LMWH. Hb remained relatively stable at 83 mg/dL. On POD5, a further reduction in PC was observed (33 × 109/L) and following hematologist advice, the patient was started on fondaparinux for suspected heparin-induced thrombocytopenia (HIT). HIT screening (enzyme-linked immunosorbent assay (ELISA) test) was performed and after it was confirmed negative, fondaparinux was discontinued and one pool of platelets was transfused. As the PC continued to drop (22 × 109/L), the patient was screened for TTP with specific testes performed under hematology guidance and advice. The tests performed included fibrinogen, D-dimers, von Willebrand factor (vWF) antigen with multimeric pattern analysis and a disintegrin and metalloprotease with thrombospondin type 1 motifs 13 (ADAMTS13) testing. While the test results were awaited the patient’s PC reduced to 12 × 109/L, despite further transfusion of two pools of platelets. Two weeks after the surgery, the diagnosis of TTP was confirmed and the patient underwent treatment with total plasma exchange (TPE) with quick recovery of her platelets to baseline level (Fig. 1). The patient from the first post-operative day to full recovery remained clinically free of signs or symptoms related to TTP.
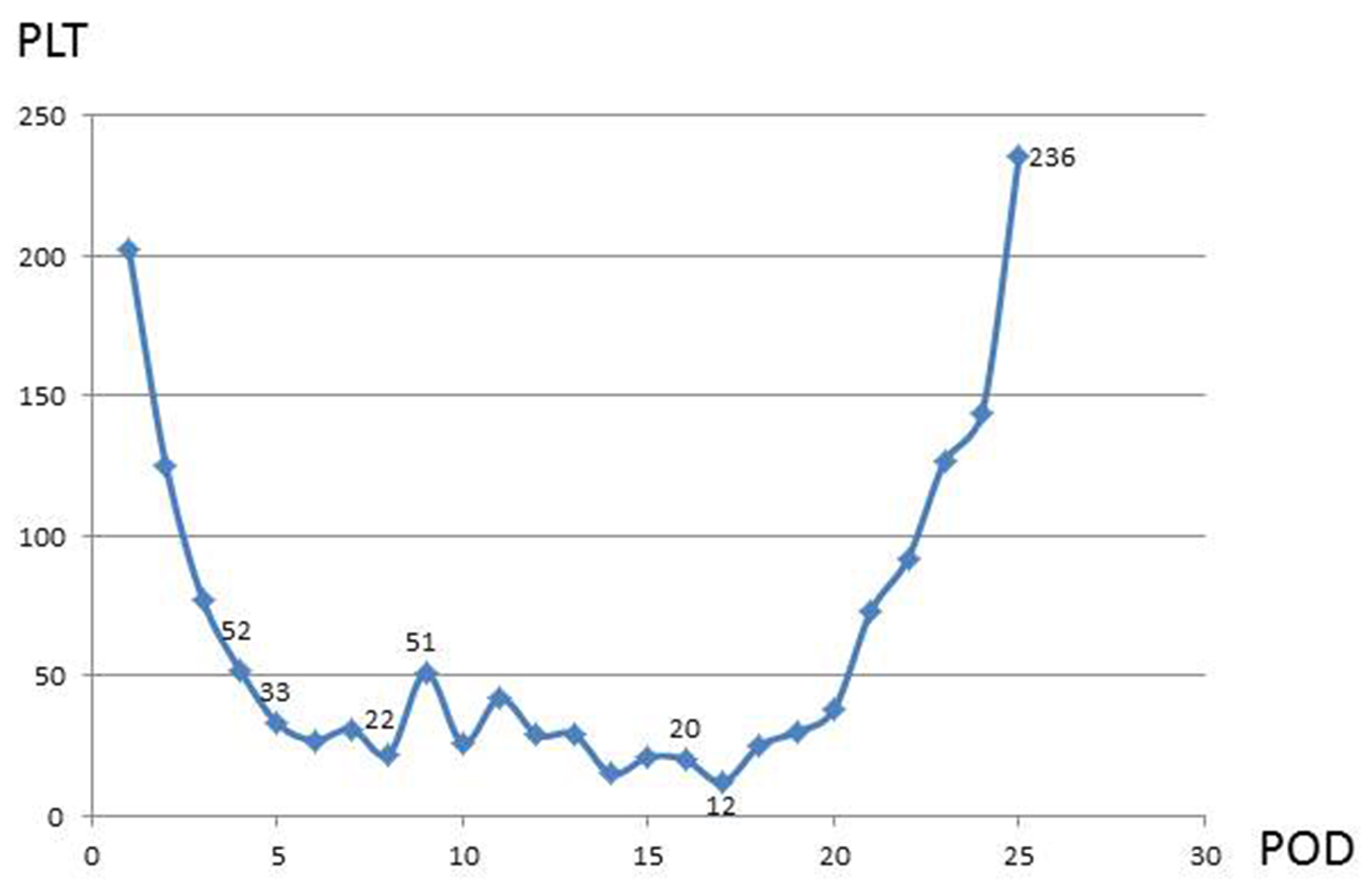 Click for large image | Figure 1. The graph shows the post-operative platelet (PLT) count fluctuation in relation to post-operative days (PODs) and therapeutic interventions. 52: POD4 PLTs count 52 (LMWH stopped); 33: POD5 PLTs count 33 (HIT screening sent); 22: POD8 PLTs count 22 (ELISA results false-negative, fondaparinux stopped, 1 pool PLTs transfused); 51: POD9 vWs protease (ADAMTS13), vWs inhibitor, Coombs, LDH sent, POD16 TTP diagnosis confirmed; 20: POD17 (plasma exchange × 6); 236: POD25 (PLTs count normalized). |
| Discussion | ▴Top |
TTP is one of the most difficult diagnoses for the clinical cardiologist to establish following cardiac surgery, due to the rarity of the disease and poor specificity of clinical and laboratory signs and symptoms. The classic pentad (thrombocytopenia, anemia, fever, neurological and renal abnormalities) is fully present in only a minority of surgical patients. Consumptive thrombocytopenia and microangiopathic hemolytic anemia (MAHA), evidenced by fragmented red cells in blood smears, and elevated serum levels of lactate dehydrogenase, which were present in our patient, provide the clinician with the most diagnostically useful laboratory signs. These abnormalities, however, may also be present in an array of other conditions characterized by disseminated thrombosis in the microcirculation (thrombotic microangiopathies) such as classic hemolytic uremic syndrome (HUS), atypical HUS (aHUS), disseminated intravascular coagulation (DIC) or sepsis. Since TTP requires specific treatment, prompt investigations are required [4, 5].
Post-operative TTP is commonly due to acquired ADAMTS13 autoantibodies causing severe ADAMTS13 deficiency. It is most often associated with cardiac or vascular surgery and has been stipulated that it results from endothelial damage. This can be secondary to a release of ultra-large vWF in the setting of low levels of vWF cleaving protease, ADAMTS13. This protease regulates the size of vWF multimers and is an important mediator of platelet adhesion. ADAMTS13 regulates vWF size by cleaving a specific peptide bond in the A2 domain. Without this regulation, accumulation of ultra-large vWF multimers leads to microvascular platelet thrombi, destroying red blood cells and platelets and compromising blood flow to vital organs such as the brain and kidneys [4-6].
The laboratory tests used to aid diagnosis include vWF antigen and multimeric pattern analysis and ADAMTS13 activity and autoantibodies. Identifying the causative antibodies may help solidify the TTP diagnosis and provide prognostic information related to response to TPE and risk of relapse [7]. Severe ADAMTS13 deficiency, defined in the literature as < 10% of normal activity, appears to have good sensitivity (about 90%) and specificity (approximately 90% or higher) for TTP, although there is some variability depending on the datasets and definitions used [8]. Studies also have shown that severe deficiency predicts good response to TPE, with approximately 80-90% of severely deficient patients responding to first-line therapies. Although severe ADAMTS13 deficiency predicts good response to TPE, it also portends a significant risk of disease relapse, which occurs in at least one-third of cases. Some patients demonstrate severe ADAMTS13 deficiency even while in clinical remission, which is likely highly predictive of relapse risk [9].
Clinical prediction scores using readily available laboratory information (creatinine, platelet count, d-dimer, reticulocyte percentage, indirect bilirubin, etc.) have proven useful for acute decision-making. The initial therapeutic regimen for acquired TTP involves immunosuppression and TPE to suppress antibody formation, decrease antibody titer and provide supplemental ADAMTS13 [10, 11]. Although confirming severely decreased ADAMTS13 activity helps establish the TTP diagnosis, therapy must start even before test results are available. In fact, clinicians determine the duration of TPE based on PC recovery and resolution of hemolysis and neurological symptoms, rather than recovery of ADAMTS-13 activity [10, 11].
Returning to our opening case, patient’s working diagnosis of acquired TTP was confirmed based on laboratory findings of severe ADAMTS13 deficiency and an ADAMTS13 inhibitor of 1.8 BU identified in pre-therapy specimens. Her initial clinical history and laboratory results largely excluded other processes associated with MAHA and thrombocytopenia, like HUS, aHUS and DIC.
As previously mentioned, thrombocytopenia is a common complication of cardiac surgery, including valve surgery. Thielmann et al found that patients in whom a low platelet count prompted testing for HIT antibodies (regardless of whether testing was positive or not) were showing high mortality, which suggests that a persistently reduced platelet count is an adverse prognostic sign after cardiac surgery [2]. Hilker et al described thrombocytopenia following AVRs with Carpentier Edwards Perimount and Sorin Biomedica Freedom SOLO bio-prosthesis [12]. Van Straten et al found that patients after AVR with Carpentier Edwards Perimount and Medtronic Freestyle stentless bio-prosthesis had significantly lower post-operative platelet count in comparison to patients receiving ATS and St. Jude Medical mechanical prostheses [13]. Ravenni et al. have observed post-operative thrombocytopenia after AVR with Sorin Freedom SOLO, Medtronic Mosaic and the Sorin Mitroflow bio-prosthesis, which was more pronounced for the Sorin Freedom SOLO valve [14].
To the best of our knowledge, thrombocytopenia secondary to TTP has never been described following AVR with trifecta bio-prosthesis. In this case report, following the diagnosis of thrombocytopenia, the patient was only screened for HIT which led to a considerable delay in diagnosing TTP, a potentially life-threatening complication that can be easily treated if diagnosed at an early stage. With this case report, we would like to not only report the first case of TTP after AVR using the trifecta bio-prosthesis, but also to recommend that patients with severe and progressive thrombocytopenia after biological AVR are screened early for TTP along with HIT, in an attempt not only to shorten the decision-making process but also to provide the appropriate therapy early.
| References | ▴Top |
- Weerasinghe A, Taylor KM. The platelet in cardiopulmonary bypass. Ann Thorac Surg. 1998;66(6):2145-2152.
doi - Thielmann M, Bunschkowski M, Tossios P, Selleng S, Marggraf G, Greinacher A, Jakob H, et al. Perioperative thrombocytopenia in cardiac surgical patients - incidence of heparin-induced thrombocytopenia, morbidities and mortality. Eur J Cardiothorac Surg. 2010;37(6):1391-1395.
doi pubmed - Marques MB. Thrombotic thrombocytopenic purpura and heparin-induced thrombocytopenia: two unique causes of life-threatening thrombocytopenia. Clin Lab Med. 2009;29(2):321-338.
doi pubmed - Naqvi TA, Baumann MA, Chang JC. Post-operative thrombotic thrombocytopenic purpura: a review. Int J Clin Pract. 2004;58(2):169-172.
doi pubmed - Chang JC, Shipstone A, Llenado-Lee MA. Postoperative thrombotic thrombocytopenic purpura following cardiovascular surgeries. Am J Hematol. 1996;53(1):11-17.
doi - Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211-225.
doi pubmed - Rieger M, Mannucci PM, Kremer Hovinga JA, Herzog A, Gerstenbauer G, Konetschny C, Zimmermann K, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106(4):1262-1267.
doi pubmed - Barrows BD, Teruya J. Use of the ADAMTS13 activity assay improved the accuracy and efficiency of the diagnosis and treatment of suspected acquired thrombotic thrombocytopenic purpura. Arch Pathol Lab Med. 2014;138(4):546-549.
doi pubmed - Starke R, Machin S, Scully M, Purdy G, Mackie I. The clinical utility of ADAMTS13 activity, antigen and autoantibody assays in thrombotic thrombocytopenic purpura. Br J Haematol. 2007;136(4):649-655.
doi pubmed - George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116(20):4060-4069.
doi pubmed - Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8(4):631-640.
doi pubmed - Hilker L, Wodny M, Ginesta M, Wollert HG, Eckel L. Differences in the recovery of platelet counts after biological aortic valve replacement. Interact Cardiovasc Thorac Surg. 2009;8(1):70-73.
doi pubmed - van Straten AH, Hamad MA, Berreklouw E, ter Woorst JF, Martens EJ, Tan ME. Thrombocytopenia after aortic valve replacement: comparison between mechanical and biological valves. J Heart Valve Dis. 2010;19(3):394-399.
pubmed - Ravenni G, Celiento M, Ferrari G, Milano A, Scioti G, Pratali S, Bortolotti U. Reduction in platelet count after aortic valve replacement: comparison of three bioprostheses. J Heart Valve Dis. 2012;21(5):655-661.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


