| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 8, Number 6, December 2017, pages 293-303
High-Density Mapping in Ventricular Tachycardia Ablation: A PentaRay® Study
Petra Maagha, d, Arnd Christopha, Henning Doppa, Markus Sebastian Muellera, Gunnar Plehnb, c, Axel Meissnera, c
aDepartment of Cardiology, Electrophysiology and Intensive Care, Klinikum Merheim, University Witten/Herdecke/Germany, Ostmerheimer Str. 200, 51109 Cologne, Germany
bDepartment of Cardiology and Angiology, Malteser-Krankenhaus St. Anna, Albertus-Magnus-Str. 33, 47259 Duisburg, Germany
cRuhr-University of Bochum, Universitatsstrasse 150, 44801 Bochum, Germany
dCorresponding Author: Petra Maagh, Klinikum Merheim, University Witten-Herdecke/Germany, Ostmerheimer Str. 200, 51109 Cologne, Germany
Manuscript submitted November 21, 2017, accepted November 28, 2017
Short title: Multielectrode Mapping Catheter
doi: https://doi.org/10.14740/cr636w
| Abstract | ▴Top |
Background: High-density mapping of ventricular tachycardia (VT) with PentaRay® (Biosense-Webster) provides high resolution with discrimination of local abnormal electrograms and slow conducting channels. We evaluate the feasibility of PentaRay® to characterize the anatomical substrate and assume an influence of the outcome despite limitations.
Methods: Over a 24-month period, 26 endocardial and four epicardial maps were obtained of 26 VT patients (18 ischemic cardiomyopathy (ICM, 69.2%) and 8 non-ischemic cardiomyopathy (NICM, 30.8%), age 65 ± 9 years). Catheter ablation (CA) was performed with the aim of transecting the isthmus. The endpoint was non-inducibility of any VT. Manual review of the maps was performed and focused on evaluating scarring, bipolar electrograms, and procedure times.
Results: In 55.6 ± 34.4 min, 1,085.9 ± 726.2 points were created. The mean ablation time was 50.8 ± 30.1 min. The endpoint was achieved in 12 patients (46.2%). The mean dense scar area and the mean patchy scar area were 49.4 ± 51.8 cm2 (range 0 - 190 cm2) and 14.7 ± 14.9 cm2 (range 0 - 110 cm2), respectively. Analyzing the learning curve, we found a tendency in decreasing procedure times. During the course of follow-up treatment averaging a 14-month period, device interrogation showed that 17 patients (65.4%) had remained free of any arrhythmia recurrence.
Conclusion: The high-density maps with PentaRay® were safely created in a short period of time. Our manual review of the maps reveals limitations of current annotation criteria; nevertheless, medium-term outcomes were encouraging. Further prospective studies are required to validate our findings in a larger cohort of patients.
Keywords: Ventricular tachycardia; Mapping; PentaRay®; Follow-up
| Introduction | ▴Top |
Catheter ablation (CA) of ventricular tachycardia (VT) in the treatment of structural heart disease (SHD) with the primary goal of the interruption of critical areas with slow conduction offers the possibility of improving patient quality of life, reducing mortality as well as painful defibrillator interventions and heart failure hospitalizations [1-3]. High-density mapping seems to have a substantial impact on clinical outcomes subsequent to having performed CA. This may be explained by a more mechanistic understanding of slow conduction areas during sinus rhythm (SR), and therefore, by being able to more efficiently target the entry site to interconnected channels [4].
The 20-pole PentaRay® catheter (Biosense-Webster Inc., Diamond Bar, CA, Fig. 1a-f) is one of the newer multipolar mapping catheters for VT ablation, and is assumed to be accurate in mapping and guiding ablation of VT [5-8]. Knowing the advantages offered by the catheter, we hypothesize that VT ablation with the PentaRay® yield to reduction of VT recurrences in follow-up due to its high resolution. With a manual review of the “on-the-fly” electrograms annotated automatically during the procedure, we discuss limitations of automatic algorithms for annotation.
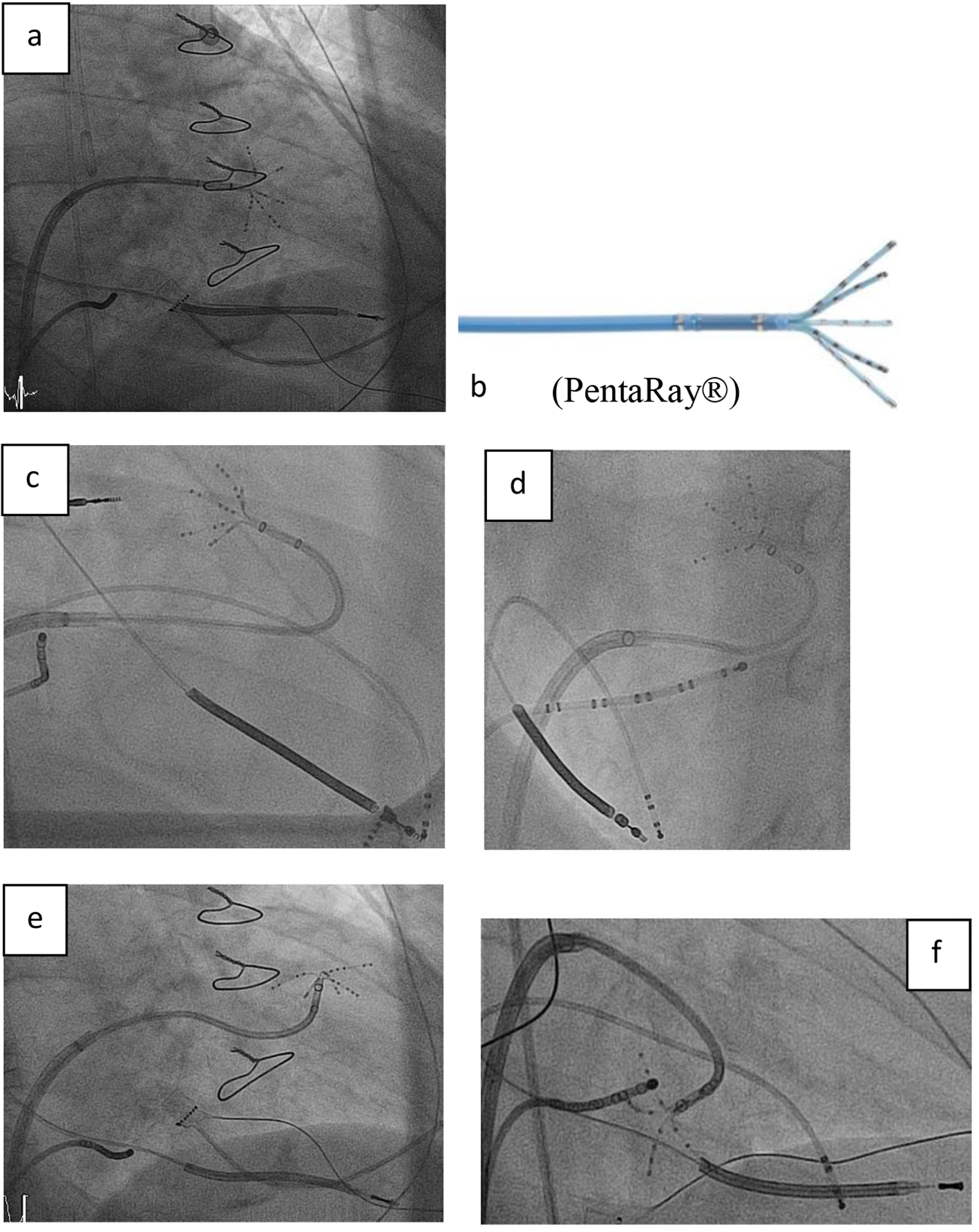 Click for large image | Figure 1. (a, b) Right anterior oblique (RAO) view showing the steerable sheath (large curve Agilis™, SJM), and the 20-pole steerable catheter PentaRay® with 1 mm electrodes distributed over five soft, radiating spines (2-6-2 mm interelectrode spacing) entering the left ventricle by passing the mitral valve. (c-f) Fluoroscopy images in RAO and left anterior oblique (LAO) view showing the various 180° of unidirectional flexion of the PentaRay® with its soft and very flexible branches allowing reaching any region in the left ventricle. |
| Materials and Methods | ▴Top |
Study population
In a single-center study taking place from November 2014 to November 2016, 26 patients with SHD (either ischemic cardiomyopathy (ICM) or non-ischemic cardiomyopathy (NICM)) undergoing VT ablation with the PentaRay® were retrospectively analyzed. Indication consisted of documented episodes of repetitive sustained VT resistant to antiarrhythmic drug therapy and requiring external cardioversion, implantable cardioverter defibrillator (ICD) antitachycardia pacing or shocks.
Electrophysiological study and left ventricular access
The electrophysiological study and ablation were conducted by collectively administering midazolam, propofol and sufentanil. Intra-arterial blood pressure monitoring and digital pulse oximetry were continuously performed. ICD therapies were inactivated. The mode of access to the left ventricle (LV) was performed at the operator’s discretion: by transseptal and/or retrograde routes with or without percutaneous subxiphoid pericardial access for additional epicardial mapping. VT induction was attempted at baseline with programmed ventricular stimulation (PVS) from the right ventricle (RV) apex at three drive trains (500, 400 and 330 ms, provided this was hemodynamically tolerated), with up to three extra-stimuli decremented to ventricular refractoriness or 200 ms.
Electroanatomical mapping (EAM) with PentaRay® and acquisition of bipolar electrograms
An EAM of the left ventricle (LV) was performed either in SR or in paced rhythm. The catheter was introduced either retrogradely - using standard sheaths - or transeptally/epicardially - using the Agilis NxT steerable introducer (St. Jude Medical). A maximum fill threshold of 10 mm was established to fill the LV cavity, and sufficient sampling of the low-voltage area was performed to obtain a fill threshold < 8 mm. In order to create the voltage map, regular bipolar cut-off of < 0.5 mV was used to determine scarring, 0.5 - 1.5 mV border zone, > 1.5 mV normal tissue [9], and 0.5 - 1.0 mV epicardial scar border zone [10]. “Patchy patterns” of electroanatomical scarring were defined as < 2 low-voltage areas separated by areas of preserved voltage (> 1.5 mV) [11]. During point acquisition, all sites with either late potentials (LP as described by Vergara et al [12]), or local abnormal ventricular activity (LAVA, as described by Jais et al [8]) within or adjacent to areas of dense scarring were identified and marked accordingly on the map. Pacing maneuvers were typically performed at the border zone of the scar where LAVA is more often found hidden within the far-field ventricular electrogram. Pace-mapping (PM) was also performed at sites within channels, in defined areas containing any abnormal electrogram, as well as in regions in which VT exits were suspected - based on 12-lead ECGs, when available. In this context, we used the definition of channels described by Hsia et al [13]: 1) a path demonstrating contiguous electrogram recordings with voltage amplitude higher than that of the surrounding areas as evidenced by distinct voltage map color differences and 2) a path involving at least two distinct, orthodromically activated sites within the reentrant circuit as defined by entrainment mapping. Before locations were tagged on the EAM, stability and adequate splaying of the PentaRay® splines over the endocardium was fluoroscopically confirmed, and ventricular ectopic beats were vigilantly excluded. In the case of hemodynamically stable VT, the VT was mapped to identify the site of the earliest ventricular activation as well as abnormal presystolic ventricular potentials, if any were present. Whenever feasible, entrainment mapping was performed at sites with abnormal electrograms, long S-QRS latencies, and paced QRS morphology coinciding with VT [14]. Tachycardia was terminated by overdrive pacing, ablation or by external/internal cardioversion. At the end of the procedure, the maps were stored for offline calculation of scar areas and bipolar electrograms.
Radiofrequency ablation and procedural endpoint
Radiofrequency current was delivered using an irrigated-tip catheter (Thermocool, Biosense-Webster) with a power setting of between 30 and 50 W, and a temperature limit of 43 °C. In patients with inducible VT and hemodynamic stability, ablation was performed at the site of the critical isthmus based on entrainment maneuvers and timing of the local EGM during VT. In the case of non-inducibility or hemodynamic instability, a substrate-based ablation approach with elimination of all annotated points (LPs and LAVAs) was performed. Channels identified inside dense scarring were connected to the surrounding tissue (Fig. 2). Linear ablation lesions were placed to transect the VT isthmus. To evaluate the procedural success, PVS was repeated subsequent to CA: complete success was defined as VT induction at baseline and no inducible VT subsequently to ablation; partial success as 1) abolition of ≥1 clinical VTs with other VTs remaining inducible and 2) non-inducibility in PVS at baseline or when we abandoned PVS subsequently to ablation due to non-inducibility or hemodynamic instability. A failure was considered when it was not possible to eliminate the clinical VT. A remapping at the end of the procedure to identify residual LAVAs and/or LPs was not performed.
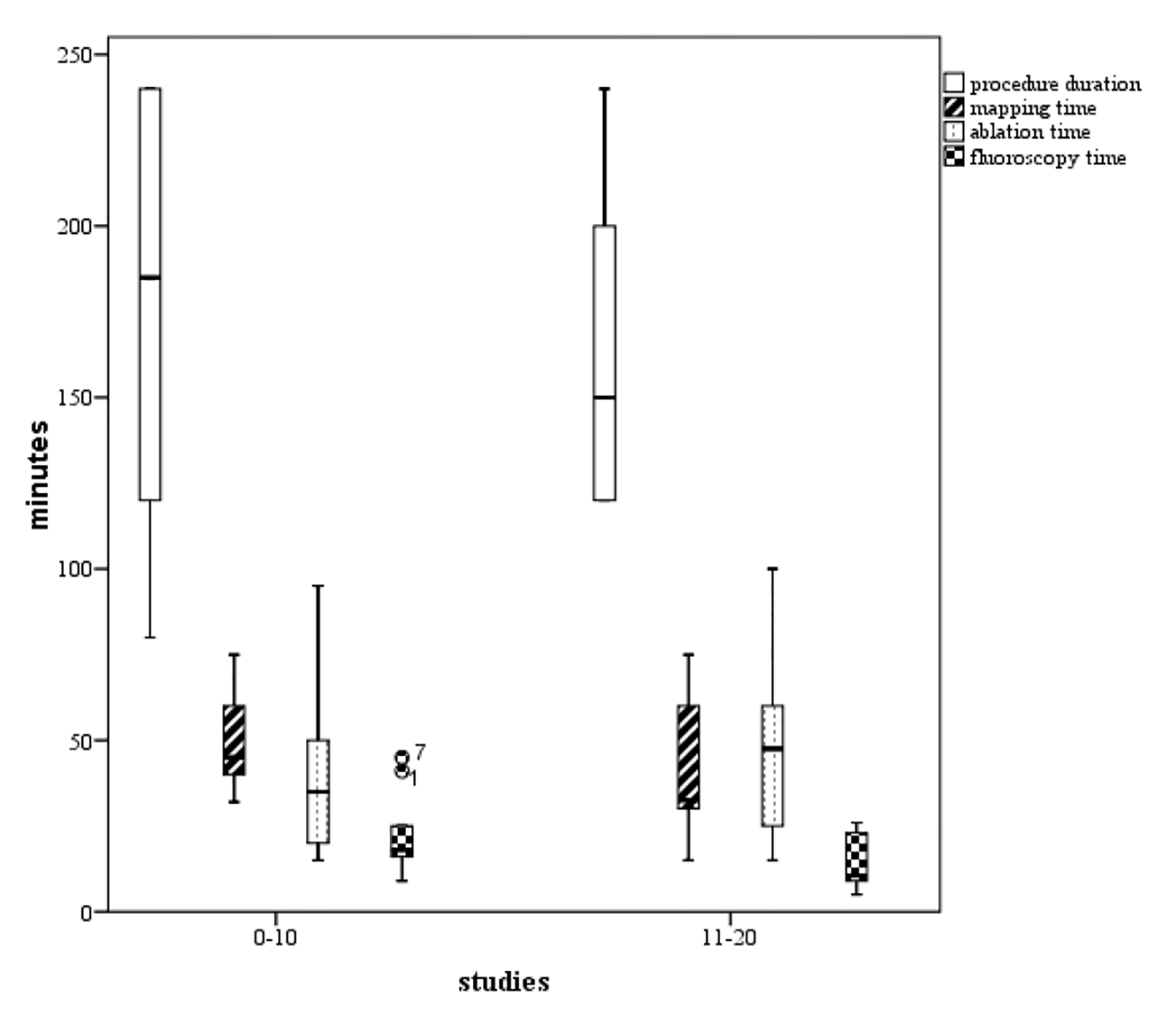 Click for large image | Figure 2. Boxplots of the procedure duration, mapping time, ablation time, and fluoroscopy time. We found a tendency to increased competence with the technology (P not significant), comparing phase 1 (the initial 10 cases) representing the learning curve, and phase 2 (the next 10 cases). |
Postprocedural EAM analysis
All EAM was reviewed offline. Each recorded electrogram - its morphology and timing in relation to QRS complex in the surface ECG - was verified by two experienced electrophysiologists. The description of the LV focused on the extension of scarring based on low-voltage areas and bordering zones. The total scar area, the dense scar and the “patchy pattern scar” were all calculated using the measurement tool included in the CARTO®3 software. The total number of points on each map - as well as the number of LPs, LAVAs and channels - was documented. We completed the data by including and entering the procedure duration and fluoroscopy times - both sets of recordings having started with the transfemoral puncture and ceasing after the last PVS. Furthermore, mapping and ablation times were included when available. The mapping time included both the creation of “geometry” as well as voltage acquisition.
Follow-up
All patients had had or received ICD, and were clinically monitored at 3 monthly follow-ups which included device interrogation. Recurrence was defined as the occurrence of sustained VT and/or appropriate ICD therapies. Antiarrhythmic therapy and/or the decision to conduct a re-ablation procedure were at the discretion of the treating physician.
Statistical analysis
Baseline parameters are presented as mean ± SD, proportion, and median with interquartile range for continuous, categorical, and count variables, respectively. Comparisons were done using a Student’s t-test, χ2 test (or Fisher exact test, where applicable), or Mann-Whitney U test, as appropriate.
| Results | ▴Top |
Baseline characteristics
The majority of the 26 patients (aged 65 ± 9 years) were male (84.6%) with characteristics of advanced SHD, including markedly depressed ventricular function. Out of the 26 patients, 25 patients were carriers of a device: 21 patients (80.8%) had an ICD, and four patients (15.4%) had had cardiac resynchronization therapy (CRT). More than half of the patients (14 patients, 53.9%) were NYHA ≥ 3. Further, 50% of the patients had already had ≥ 2 VT ablation procedures in the past. The ejection fraction (EF) averaged 28.1±11.5%. Etiologies of SHD included 18 patients with ICM (69.2%), and eight with NICM (30.8%). The infarction area of the ICM patients was anterior/septal in 50.0%, inferoposterior in 11 (42.3%), and lateral in two (7.7%) of the cases. Etiologies included two idiopathic, one arrhythmogenic right-ventricular dysplasia, three myocarditis and two toxin-induced NICM types. More patients with ICM had a history of electrical storm or incessant VT than those with NICM (Table 1).
 Click to view | Table 1. Clinical Characteristics of PentaRay® Patients |
Mapping and ablation procedure
Transseptal, retrograde aortic, and epicardial approaches were performed in 24 (82.3%), six (23.1%), and four cases (15.4%), respectively. We aimed to do PVS at baseline in all patients, but we remained to perform PVS in one patient with higher grade aortic stenosis and one patient with hemodynamic instability at baseline. Thus, PVS was done in 24 patients (92.3%). In 17 out of these 24 patients (65.4% of the total cohort), VT was inducible; in seven patients, VT was not inducible. The induced VTs showed a cycle length of 366.2 ± 90.5 ms on average. In seven ICM patients, multiple VT (ranging from 2 to 5) was observed. During VT, entrainment mapping could succeed in three patients (11.5%), and led to VT termination in two. In the remaining 14 patients (46.2% of the total cohort), mapping during VT was impossible to perform due to either non-inducibility (in two patients, as mentioned above), hemodynamic intolerance (in seven patients), or conversion to another VT after VT induction (in five patients). PVS after ablation was performed in only 12 patients from those 17 patients induced at baseline. These 12 patients were not inducible after ablation. They were considered as complete success with VT inducibility at baseline and non-inducibility after ablation according to the method section (46.2%). The procedure success of patients with non-inducibility after ablation was considered as partial success (14 patients, 53.9%). No failure occurred. No patients experienced pericardial bleeding; there were no strokes, phrenic palsies, coronary injuries, or procedure-related deaths. The procedure lasted 175.4 ± 52.0 min on average (ranging from 80 to 300 min), the mapping time averaged 55.6 ± 34.4 min (ranging from 15 to 160 min), and the fluoroscopy time averaged 50.8 ± 30.1 min (ranging from 15 to 130 min). For further details, see Table 2.
 Click to view | Table 2. Procedural Data of the PentaRay® Patients |
Learning curve of the PentaRay®
Figure 3 shows boxplots of the procedure duration as well as the times of mapping, ablation, and fluoroscopy. The learning curve consisted of two unique phases, whereby phase 1 (the initial 10 cases) represented the initial learning curve. Phase 2 (consisting of cases 11 - 20) experienced increased competence with the technology chosen to use: procedure duration, mapping and fluoroscopy times in phase 1 took 174, 49.1, and 21.8 min compared to the times of those patients belonging to the phase 2 group taking 161, 39.5, and 13.5 min, respectively (P values not significant). In comparison, the ablation time increased from 43.5 min in the phase 1 group to 47.0 min in the subsequent phase 2 group (P value not significant).
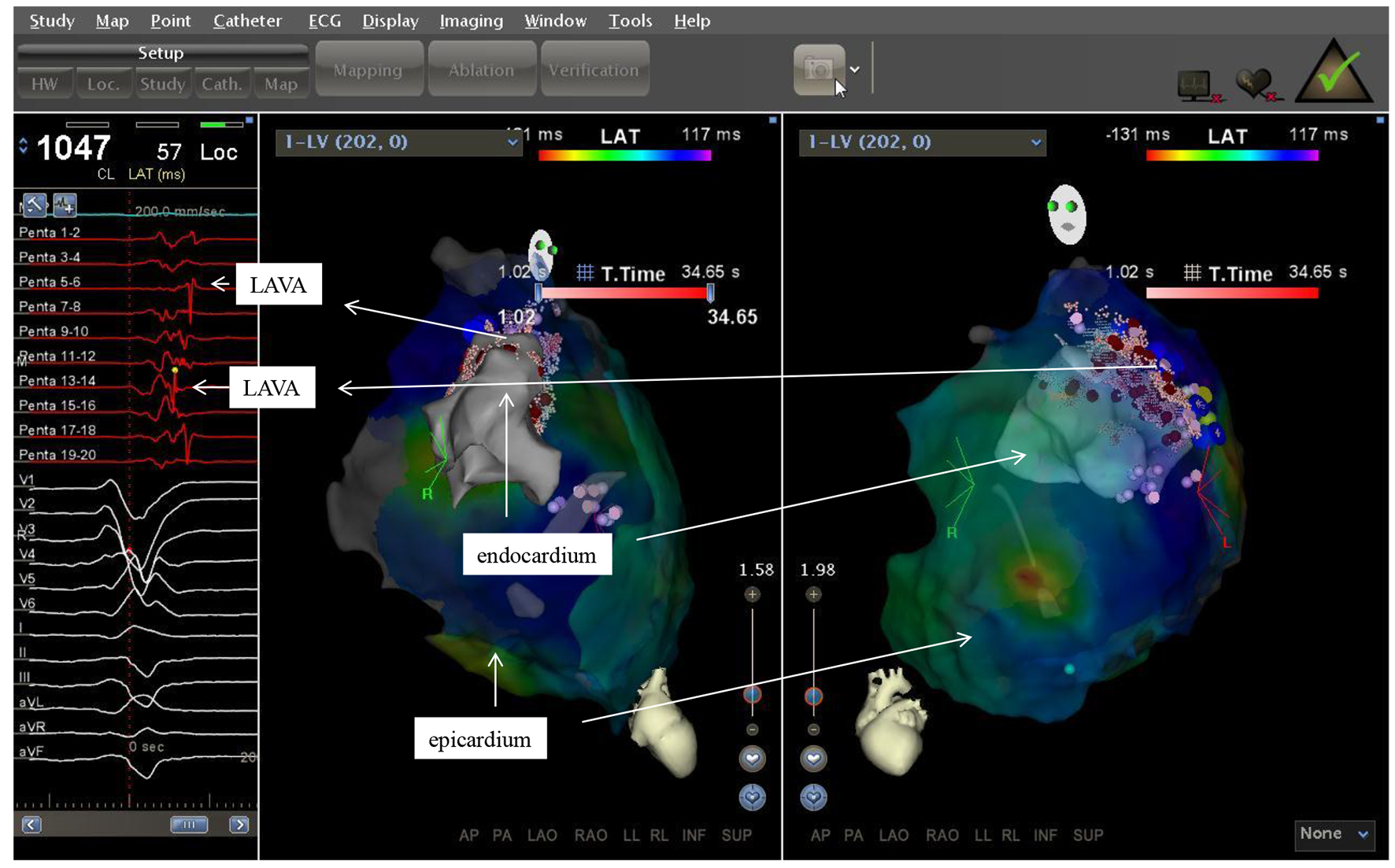 Click for large image | Figure 3. Two electroanatomic map of a patient with non ischemic cardiomyopathy. Left lateral and right anterior oblique views of the epicardium and a part of the left ventricle are depicted. Substratemap of the pericardium obtained with the PentaRay® was set at 45% transparency, so that each recorded bipolar electrogram, its morphology and timing in relation to QRS complex could be related to the underlying tissue. |
Postprocedural EAM analysis
Scarring
A total of 30 EAM were reviewed offline by two experienced electrophysiologists. In 26 endocardial maps and four epicardial maps, a mean number of 1,085.9 ± 726.2 mapping points having been created in 55.6 ± 34.4 min was determined and analyzed. To describe the ventricle and the abnormal substrate, we first measured the number of scar and the size of each scar. The total number of scars visible in 30 EAMs ranged between 1 and 5. We found the majority of patients (57.6%) having more than one area of scarring. A maximum of two dense scars was accounted for in patients with ICM; a maximum of five patchy scars were accounted for in patients with NICM. The average absolute surface area of dense scarring and the mean patchy scarring area was 49.4 ± 51.8 cm2 (ranging from 0 to 190 cm2) and 14.7 ± 14.9 cm2 (ranging from 0 to 110 cm2), respectively. Dense scarring averaged 15.3 ±16.5 cm2; the mean percentage of patchy scarring was 1.7 ± 5.1 cm2. The requirement that VT was related to the scarring on EAM was fulfilled in our study; the suspected local VT exits, firstly based on 12-lead ECGs, were often able to be confirmed by EAM.
Bipolar electrograms
In the following step, we verified each recorded bipolar electrogram, its morphology and timing in relation to the QRS complex in the surface ECG and correlated it to the underlying tissue by setting the transparency in CARTO®3 at 100% (Fig. 4). Noise, artifacts, and near-field versus far-field specifications were rigorously analyzed and excluded, when necessary. The points that had to be discarded as they were judged to be noise/far-field/artifact were not counted, so that we cannot relate them to all the points acquired automatically in the EAM. The majority of abnormal EGMs was recorded in areas of scarring/low-voltage, i.e. fractionated potentials, double potentials, LPs and LAVAs. LPs were recorded in 24 patients (92.3%) and LAVAs in 20 patients (76.9%). The number of LPs in all patients averaged 36.4 ± 29.9 (ranging from 0 to 116) - higher than the number of LAVAs which averaged 11.0 ± 11.3 (ranging from 0 to 39). We identified channels in 73.1% of patients. Fractionated potentials were present in all patients (hereby ranging from 4 to 93 and averaging 28.5 ± 20.9). It was remarkable how difficult it sometimes was to distinguish between the bipolar signals, although the most important signals are clearly defined and pictured in the literature. In the offline analysis without pacing maneuvers, it was not always possible to clearly identify LAVAs from “mere” fractionated potentials. By setting the transparency in CARTO®3 at 100%, we attempted to determine the location of each point in the map, thereby serving to better guide our interpretation. Furthermore, we aimed adjusting the color scale display in order to ultimately identify potential channels within dense scarring [13, 15]. Nevertheless, by increasing the transparency and analyzing the annotated bipolar electrograms, it was remarkable how many points were missing by the operator during the ablation procedure itself, especially inside the dense scar area.
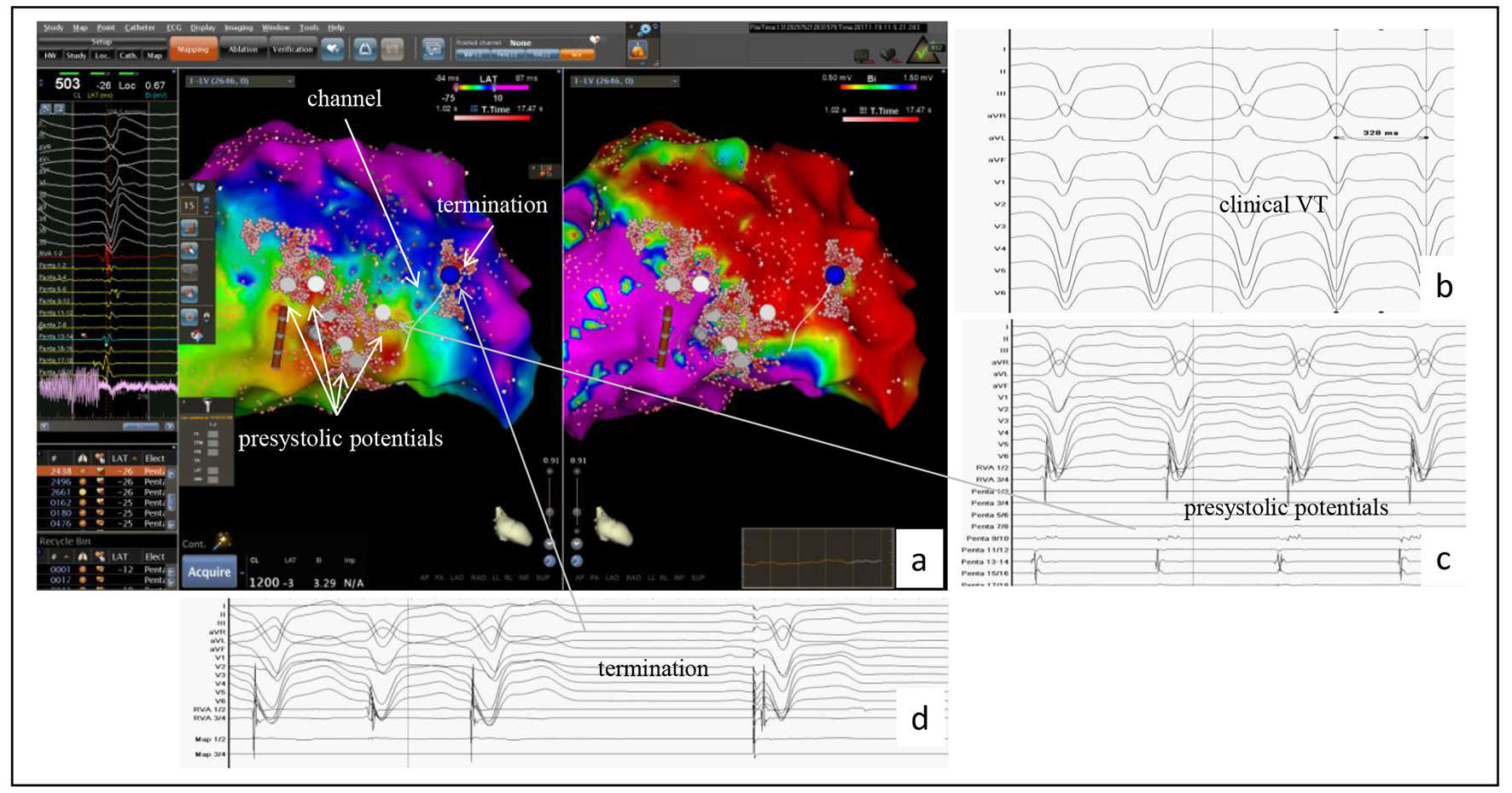 Click for large image | Figure 4. Example of the accuracy of the PentaRay® mapping catheter identifying a channel in a patient with a prior anterior wall myocardial infarction. (a) CARTO®3-guided electroanatomic map (EAM) of the left ventricle: activation map left sided, in which the area with the latest activation appears in blue, and the voltage map right sided with identification of a large dense scar in the anterior wall; to the left the bipolar signals of LAVAs at the center showing the latest activities suggesting a channel identified by the PentaRay® (white line in the EAM). This channel yields from inside the dense scar into the border zone and was identified as ablation target. (b) At baseline, VT was successful induced and well tolerated (CL 330 ms). (c) During VT, in the border zone, prasystolic fractionated signals but not mid-diastolic potentials (gray arrow) were recorded with the PentaRay® and annotated on the EAM (white points and gray arrow). Mapping the dense scar, the channel was then found inside dense scar and also annotated on the EAM (EAM, arrow). (d) VT terminates under radiofrequency ablation (blue point in the maps); the first beat is a paced rhythm. |
Follow-up
One patient was lost during follow-up. The only patient without a device received an ICD following the ablation procedure. During the course of follow-up lasting 14 months on average, 25 patients (97.2%) were monitored with device interrogation. Freedom from VT recurrence was achieved in 17 patients (65.4%, one patient with NICM). In eight patients (30.8%), we found VT recurrences during device interrogation (for further details, see Table 3).
 Click to view | Table 3. Follow-Up of the PentaRay® Patients |
| Discussion | ▴Top |
The main findings of our study are as follows: 1) the catheter is feasible and safe in a real world scenario; 2) the catheter serves to acquire an accurate and high-density map of the abnormal substrate in a short time; 3) LPs and LAVAs can be quickly targeted; 4) channels can be identified. Our study offers a detailed description of the clinical utility of the multielectrode mapping catheter PentaRay® during VT ablation in a small series of patients with severe ICM and NICM (the half of them had experienced ≥ 2 VT ablations in the past), specifically designed with this purpose. The focus was not on the comparison of multielectrode mapping catheters with linear catheters as other authors did recently [5, 16, 17]. In our opinion, the only comparable study in the current literature focusing the same issue of clinical utility of the PentaRay® was recently published by Cano et al [18]. In addition, we offer an offline analysis of the EAM created with the PentaRay®. We observed that despite limitations of “on-the-fly” electrograms annotated automatically during the procedure, the detailed mapping seems to be associated with achieving a satisfied clinical outcome.
Study-based feasibility, safety and accuracy of the PentaRay® mapping catheter
Using standard sheaths, the PentaRay® could easily be advanced into the LV. We preferred the transseptal approach (chosen in 24 patients, 82.3%), and as already described [8], entering the LV with the smooth catheter by crossing the mitral valve was feasible and safe - with no complications attributable to the catheter. We had always aimed to represent the whole cavity of the left ventricle. To picture well the anterior wall of the LV, we pushed in case of a transseptal access the Agilis NxT steerable introducer carefully forward and deep in the LV. Due to the high torque capabilities and the nearly unlimited maneuverability of the PentaRay®, loops against the anterior wall were easily done without traumatic injuries (Fig. 1). The best regions to reach with the PentaRay® were the lateral wall and the septum, regardless of the access. If some regions could not be reached, we changed the access from transseptal to retrograde and vice versa. A large number of points (1,085.9 ± 726.2 mapping points, produced in 55.6 ± 34.4 min) were simultaneously and quickly acquired - with high-resolution and clean (noise-free) electric signals. The sharpness of the bipolar electrograms not only facilitated the identification of LPs and LAVAs, but even channels as well (Fig. 2). Due to the fact that the PentaRay® covers a surface diameter of > 7.0 cm2 and offers the possibility to pace from any electrode, we accelerated the time allocated for analyzing the signals during the procedure. The ability to detect scarring, patchy scars and corresponding border zones was impressive. As described in the literature [19], EAM enables to identify scars in so-called NICM, the very heterogeneous group of myocardial diseases - including multiple etiologies, but also frequently idiopathic conditions.
Analyzing the learning curve with the PentaRay®, we found a tendency in decreasing the total procedure duration, fluoroscopy and mapping times. Hence, we could shorten the procedure duration by 13 min (in mean 174 min starting with the PentaRay®, and 161 min in the latter cases), the mapping time by 10 min (in mean 49.1 and 39.5 min, respectively), and the fluoroscopy time by 8 min (in mean 21.8 and 13.5 min, respectively). Thus, our procedure duration and mapping times are still below those of Cano et al [18], who needed 189 ± 56 min for the procedure and 44 ± 20 min for mapping. As has been emphasized recently [18, 20], the reduction of mapping and procedural time in this context is of great value considering that the hemodynamic stability of these patients may be easily disrupted with longer procedures. Recent multicenter VT ablation studies have reported mean VT ablation procedure times ranging from 240 to 263 min, which are significantly longer when compared with the procedural times described in our series [21, 22].
Patients’ outcome
We favored a strict approach: complete success was only achieved with the inducibility at the beginning of the procedure and non-inducibility subsequent to ablation. For which reason a patient was not induced or not inducible before or after ablation, we considered the absence of PVS as partial success. Thus, complete success was achieved in less than half of the patients (46.2%). Even though we succeeded in inducing a high percentage of patients with PVS prior to ablation (24 patients, 92.3%), only 17 of them had had inducible VTs (65.4%). Due to different reasons, only 12 patients experienced PVS after ablation (46.2%). We underscore with our results the discussion of Tung et al [23], who recently described the limitations associated with achieving acute procedural success with PVS. It remains to discuss if a strict approach concerning follow-up consisting of 100% device interrogation perhaps better reflects the acute successful ablation rate than PVS did. It was determined that 17 patients (65.4%) had remained free of any arrhythmia recurrence in a 14-month follow-up.
Online and offline analysis of intracardiac signals
In brief, the EAM created with CARTO®3 consists of “electrical points” sampled by the mapping catheter “on-the-fly” through having contact with the anatomical structure. One of the major challenges of multipolar mapping is how to handle “on-the-fly” electrogram analysis during the procedure. One must be able to visualize and consume all the given information in a convenient manner to facilitate ablation. While automated software can exclude internal points, it has yet to provide additional well validated means to automatically exclude noise and artifact without operator intervention, or to otherwise identify high value or high yield electrogram characteristics to focus ablation efforts. Current annotation criteria might be limited and imprecise, especially in fractionated low voltage signals in scarred myocardium. The question remains whether or not the accuracy of multipolar mapping catheters and software are comparable to human interpretations. According to our observation, in a recent study with a 64-pole new basket catheter [24], manual electrogram review using the “virtual probe” confirmed that 100% automatically detected LP could be confirmed in only 69%; the remaining LP were annotated incorrectly due to an artificial signal (“false positive”). As seen in our manual review after the procedure with two observers, verifying the high number of points in high-density mappings is time consuming and difficult as well; impossible to perform such a strict analysis during the procedure - it might distract the intraprocedural workflow. Thus, automatic algorithms for annotation and display on the 3D map are crucial. New annotation algorithms might overcome this limitation in future [24].
Limitations
The study consisted of a small sample size and had no pre-specified protocols for VT. However, this is a consecutive series of patients being treated in a university hospital that reflects “real world” experience in the truest sense. The advantage of measuring the area of dense and patchy scars is questionable, as we did not correlate the scar extension with other imaging modalities. In our sample size, details of the myocardial infarction history were lacking. This information would have been helpful in order to characterize the abnormal substrate. During the procedure, artifacts and noise were manually excluded; nevertheless we decided to exclude further bipolar electrograms in our offline analysis, but we did not count them. A comparison of our results with point-by-point VT ablation seems not reasonable due to the limited number of patients involved and their heterogeneity regarding their SHD.
Conclusions
In this initial experience of 26 consecutive patients undergoing ablation for ventricular arrhythmias, mapping - exclusively using the PentaRay® mapping catheter - was fast, safe and effective in all patients. Despite limitations, the PentaRay® permits a detailed anatomic characterization of the endocardial substrate by systematically analyzing the local electrogram voltage characteristics that correlate with documented components of the reentrant circuit. Acute success was high and medium-term outcomes were encouraging. Further prospective studies are required to validate our findings in a larger cohort, including head-to-head comparisons with other contemporary mapping systems, e.g. ultra-high resolution mapping with reliable automatic point annotation using 64 electrodes (Rhythmia™, Boston Scientific, USA).
Abbreviations
CA: catheter ablation; CABG: coronary artery bypass graft; CC: slow conducting channels; CRT: cardiac resynchronization therapy; EAM: electroanatomical mapping; ECG: electrocardiogram; EF: ejection fraction; ICD: internal cardioverter defibrillator; ICM: ischemic cardiomyopathy; LP: late potentials; LAVA: local abnormal ventricular activity; LV: left ventricle; NICM: non-ischemic cardiomyopathy; NYHA: New York Heart Association; PVS: programmed ventricular stimulation; RV: right ventricle; SHD: structural heart disease; SR: sinus rhythm; VT: ventricular tachycardia; 3D-EAM: three-dimensional electronical mapping
| References | ▴Top |
- Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009-1017.
doi pubmed - Dinov B, Fiedler L, Schonbauer R, Bollmann A, Rolf S, Piorkowski C, Hindricks G, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation. 2014;129(7):728-736.
doi pubmed - Santangeli P, Frankel DS, Marchlinski FE. End points for ablation of scar-related ventricular tachycardia. Circ Arrhythm Electrophysiol. 2014;7(5):949-960.
doi pubmed - Tung R, Mathuria NS, Nagel R, Mandapati R, Buch EF, Bradfield JS, Vaseghi M, et al. Impact of local ablation on interconnected channels within ventricular scar: mechanistic implications for substrate modification. Circ Arrhythm Electrophysiol. 2013;6(6):1131-1138.
doi pubmed - Tschabrunn CM, Roujol S, Dorman NC, Nezafat R, Josephson ME, Anter E. High-resolution mapping of ventricular scar: comparison between single and multielectrode catheters. Circ Arrhythm Electrophysiol. 2016;9(6):e003841.
doi pubmed - Berte B, Relan J, Sacher F, Pillois X, Appetiti A, Yamashita S, Mahida S, et al. Impact of electrode type on mapping of scar-related VT. J Cardiovasc Electrophysiol. 2015;26(11):1213-1223.
doi - Nayyar S, Wilson L, Ganesan AN, Sullivan T, Kuklik P, Chapman D, Brooks AG, et al. High-density mapping of ventricular scar: a comparison of ventricular tachycardia (VT) supporting channels with channels that do not support VT. Circ Arrhythm Electrophysiol. 2014;7(1):90-98.
doi pubmed - Jais P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, Hocini M, et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125(18):2184-2196.
doi pubmed - Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101(11):1288-1296.
doi pubmed - Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54(9):799-808.
doi pubmed - Wijnmaalen AP, Schalij MJ, von der Thusen JH, Klautz RJ, Zeppenfeld K. Early reperfusion during acute myocardial infarction affects ventricular tachycardia characteristics and the chronic electroanatomic and histological substrate. Circulation. 2010;121(17):1887-1895.
doi pubmed - Vergara P, Trevisi N, Ricco A, Petracca F, Baratto F, Cireddu M, Bisceglia C, et al. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23(6):621-627.
doi pubmed - Hsia HH, Lin D, Sauer WH, Callans DJ, Marchlinski FE. Anatomic characterization of endocardial substrate for hemodynamically stable reentrant ventricular tachycardia: identification of endocardial conducting channels. Heart Rhythm. 2006;3(5):503-512.
doi pubmed - Reddy VY, Wrobleski D, Houghtaling C, Josephson ME, Ruskin JN. Combined epicardial and endocardial electroanatomic mapping in a porcine model of healed myocardial infarction. Circulation. 2003;107(25):3236-3242.
doi pubmed - Mountantonakis SE, Park RE, Frankel DS, Hutchinson MD, Dixit S, Cooper J, Callans D, et al. Relationship between voltage map "channels" and the location of critical isthmus sites in patients with post-infarction cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol. 2013;61(20):2088-2095.
doi pubmed - Acosta J, Penela D, Andreu D, Cabrera M, Carlosena A, Vassanelli F, Alarcon F, et al. Multielectrode vs. point-by-point mapping for ventricular tachycardia substrate ablation: a randomized study. Europace. 2017.
doi - Yamashita S, Cochet H, Sacher F, Mahida S, Berte B, Hooks D, Sellal JM, et al. Impact of new technologies and approaches for post-myocardial infarction ventricular tachycardia ablation during long-term follow-up. Circ Arrhythm Electrophysiol. 2016;9(7):e003901.
doi pubmed - Cano O, Plaza D, Sauri A, Osca J, Alonso P, Andres A, Sancho-Tello MJ, et al. Utility of high density multielectrode mapping during ablation of scar-related ventricular tachycardia. J Cardiovasc Electrophysiol. 2017;28(11):1306-1315.
doi pubmed - Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K, Yu R, et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm. 2015;12(9):1997-2007.
doi pubmed - Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S, Hutchinson MD, Supple G, et al. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol. 2015;8(1):68-75.
doi pubmed - Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375(2):111-121.
doi pubmed - Di Biase L, Burkhardt JD, Lakkireddy D, Carbucicchio C, Mohanty S, Mohanty P, Trivedi C, et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol. 2015;66(25):2872-2882.
doi pubmed - Tung R, Josephson ME, Reddy V, Reynolds MR, Investigators S-V. Influence of clinical and procedural predictors on ventricular tachycardia ablation outcomes: an analysis from the substrate mapping and ablation in Sinus Rhythm to Halt Ventricular Tachycardia Trial (SMASH-VT). J Cardiovasc Electrophysiol. 2010;21(7):799-803.
doi - Nuhrich JM, Kaiser L, Akbulak RO, Schaffer BN, Eickholt C, Schwarzl M, Kuklik P, et al. Substrate characterization and catheter ablation in patients with scar-related ventricular tachycardia using ultra high-density 3-D mapping. J Cardiovasc Electrophysiol. 2017;28(9):1058-1067.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


