| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 2, April 2024, pages 99-107
Optimizing Patient Selection for Physiological Pacing in Bradyarrhythmia: Factors Associated With High Ventricular Pacing Burden
James Manniona, e , Kathryn L. Hongb, Amy Hennesseya, Anna Clearya, Anand Subramaniyana, Conor Sheahana, c, Kathleen E. Bennettd, Richard Sheahana, c
aElectrophysiology Department, Beaumont Hospital, Beaumont, Dublin 9, Ireland
bCambridge University Hospitals NHS Foundation Trust, Cambridge, Cambridgeshire, CB2 0QQ, UK
cRoyal College of Surgeons in Ireland, University of Medicine & Health Sciences, Dublin 2, Ireland
dData Science Centre, School of Population Health, RCSI, University of Medicine & Health Sciences, Dublin 2, Ireland
eCorresponding Author: James Mannion, Electrophysiology Department, Beaumont Hospital, Dublin 9, Ireland
Manuscript submitted November 29, 2023, accepted March 5, 2024, published online April 15, 2024
Short title: Factors Associated With High RV Pacing
doi: https://doi.org/10.14740/cr1598
| Abstract | ▴Top |
Background: Right ventricular (RV) pacing is established as the most common ventricular pacing (VP) strategy for patients with symptomatic bradyarrhythmia. Some patients with high VP burden suffer deterioration of left ventricular (LV) function, termed pacing-induced cardiomyopathy (PICM). Patients who pace > 20% of the time from the RV apex are at increased risk of PICM, but independent predictors of increased RV pacing burden have not been elucidated in those who have a permanent pacemaker (PPM) inserted for bradyarrhythmia.
Methods: We aimed to identify factors that are associated with increased VP burden > 20%, hence determining those at risk for resultant PICM. In this retrospective cohort study, we identified the most recent 300 consecutive cardiac implantable electronic device (CIED) implants in our center and collected past medical history, electrocardiogram (ECG), echo, medication and pacemaker check data.
Results: A total of 236 individuals met inclusion criteria. Of the patients, 35% had RV pacing burden < 20%, while 65% had VP burden ≥ 20%; 96.2% of patients with complete heart block (CHB) paced > 20% (P = 0.002). Utilization of DDD or VVI (75.2% and 89.2% of patients, respectively) without mode switch algorithms was associated with VP > 20% (P < 0.001). Male or previous coronary artery bypass grafting (CABG) patients also statistically paced > 20%. Other factors trending towards significance included prolonged PR interval, atrial fibrillation or more advanced age.
Conclusion: High-grade atrioventricular (AV) block was associated with an RV pacing burden > 20% over 3 years but this was not consistent in patients with only transient episodes of high-grade AV block. We found a significant association between high VP% and male sex, previous CABG and the absence of mode switching algorithms.
Keywords: Left bundle branch area pacing; Physiological pacing; Preserved LV function; Pacemaker induced cardiomyopathy; RV pacing burden; Risk stratification
| Introduction | ▴Top |
Implantation of pacing device leads targeting initial activation of the right ventricle (RV) is a well-established therapeutic strategy for patients with bradyarrhythmia. However, patients who require frequent pacing via the RV lead are at risk of developing deleterious effects on left ventricular (LV) function, commonly termed pacing-induced cardiomyopathy (PICM) [1-3]. This, in turn, may also lead to challenging consequences such as increased appropriate therapies from implantable cardioverter defibrillators (ICDs) [4]. Mitigation of superfluous RV pacing is therefore of foremost priority for treating physicians and is a key tenet of proprietary algorithms amongst research and industry innovation [5].
It has not been fully elucidated as to why some patients develop cardiomyopathy, while others do not. For example, pre-implant electrocardiogram (ECG) studies have demonstrated limited predicting power [6]. Meta-analyses have identified broader native QRS, reduced left ventricular ejection fraction (LVEF) at time of implant, or increased LV end diastolic diameter as predicting factors [3, 7]. Other meta-analyses have found that older age or male sex contributes to PICM [3]. Importantly, higher RV pacing burdens of > 20% have been repeatedly found to be an independent predictor of PICM in multiple large studies [1, 8]. While the exact burden of RV pacing associated with PICM has been associated with several thresholds, those who pace > 20% of the time from the RV have been specifically found to be at increased risk. Studies indicate that the incidence of this cardiomyopathy is about 12.3% in those who initially had preserved LV function, requiring a cardiac implantable electronic device (CIED) for complete heart block (CHB) [1].
While the development of PICM may necessitate an upgrade to a biventricular system as the disease progresses [9], this additional management has limited benefit yielding a modest 20-30% improvement in response [10]. Implantation success is hinged on coronary venous anatomy and can lead to sub-optimal resynchronisation [11]. In order to mitigate this complication and the incidence of PICM, left bundle branch area pacing (LBBAP) has gained widespread attention as an alternative strategy. The potential benefits of LBBAP in those with cardiac resynchronization therapy (CRT) indications are evident, with comparatively narrower QRS complexes leading to rapid LV activation times and specific targeting of the intrinsic conduction pathway optimizing resynchronisation and thus, promoting reverse remodeling [12-14].
The aim of our study was to identify factors which may be associated with an increased RV pacing burden > 20% in those who have a device inserted for a bradyarrhythmia indication, to effectively guide first-line pacing strategy selection.
| Materials and Methods | ▴Top |
Study design and data source
This was a retrospective cohort study whereby the most recent 300 consecutive CIED implants in our center from 2017 to 2020 were included. We collected 3-year follow-up data on RV pacing lead percentage utilization. We included patients over the age of 18 years who had a PPM inserted for a bradyarrhythmia indication and who had follow-up in our tertiary center pacing clinic. We excluded patients who had ICD or biventricular devices inserted or those who had their follow-up in peripheral hospitals. We performed a chart review for each patient, collating patient medical data, pre-implant ECG parameters, echo features such as chamber size or valvular lesions and PPM clinic notes.
Patient medical and pacing data
We collated patient medical historical data (age, sex, cardiovascular risk factors, previous coronary intervention), medication lists (antiarrhythmics, rate lowing medication, common cardiovascular medications) and admission ECG parameters prior to PPM insertion using a standard data extraction form. ECG data included admission rhythm (sinus rhythm (SR), atrial fibrillation (AF)/flutter, junctional escape rhythm, ventricular escape rhythm), PR interval, the presence and type of AV nodal block, the presence and type of bundle branch or fascicular block, QRS duration, axis deviation and QTc (calculated via Fredericia formula). Admission ECG and pre-implant echocardiography are represented as number of patients “n” (total proportion of the group which is comprised by that variable).
Echocardiographic data comprised left ventricular systolic function (categorized broadly into normal LVEF > 50%, mildly impaired LVEF 40-50%, moderately impaired LVEF 30-40% or severely impaired LVEF < 30%), cardiac chamber sizes (normal or enlarged) and valvular lesions with a moderate severity or greater as per the departmental echo report. Echocardiograms performed during the PPM insertion admission or the 3 months prior to insertion of PPM were included. Reports were reviewed and parameters including chamber size, LV function and severity of valvular lesions were considered.
We analyzed indications for insertion (symptomatic pauses in SR or AF, symptomatic atrial bradycardia, transient high-grade AV block, sustained symptomatic Mobitz 2, sustained symptomatic CHB), pacemaker settings at time of insertion (VVI, DDD or AAI<->DDD utilizing proprietary pacing mitigation algorithms) and pacing check parameters over 3 years, most importantly RV pacing burden recorded as the percentage of total beats (ventricular pacing (VP)%). The VP% burden was calculated by combining the total number of any ventricular paced combinations. For example, atrial sensing with VP percentage was added to atrial pacing with VP for those with a dual chamber pacemaker.
Patients who had an indication of high-grade AV block such as CHB or second-degree heart block (Mobitz 2) were separated depending on whether the block was sustained up to time of implant or transiently identified on cardiac rhythm monitoring with associated symptoms. A new variable for pacemaker indication, “transient high-grade AV block”, was created and combined symptomatic patients with both CHB or Mobitz 2 indications for whom the AV block episodes resolved prior to PPM insertion.
Statistical analysis
Our cohort was stratified according to those who had a VP% burden < 20% and those with a VP% burden ≥ 20%. Descriptive analysis of the association between the VP% and independent variables described was undertaken. All continuous variables were examined for normality within the two groups using the Shapiro-wilk test of normality. If the continuous variables were normal then means (standard deviations (SDs)) were presented whereas for non-normal distributions, the median with interquartile range (IQR) were used. For comparison of continuous data between the VP% burden groups, t-tests (for normal) and non-parametric test Wilcoxon rank sum test (for non-normal data) were used. For comparisons between VP% groups for categorical data, then Chi-square tests were utilized.
Statistical analyses were performed on STATA V.17. A two-tailed P-value of < 0.05 was considered statistically significant.
Ethical approval
The study protocol was reviewed and ethically approved by the Beaumont Hospital Clinical Audit & Governance Committee (Institutional Review Board reference number CA2022/050). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patient characteristics
Of the 236 patients who met our inclusion criteria, 82 patients (35%) had a VP% burden < 20% and were placed in group 1, while 154 patients (65%) were found to have VP burden ≥ 20% (group 2). Baseline patient demographics and medications are described in Table 1.
 Click to view | Table 1. Past Medical History and Medication |
Overall, we identified more males (VP ≥ 20%: 70.8% vs. 29.2%; P = 0.021), and more individuals who had undergone coronary artery bypass grafting (CABG) (VP ≥ 20%: 80% vs. 20%; P = 0.048). Median age of implant was 77 years (IQR: 68 - 82) in VP < 20% vs. 77.5 years in VP ≥ 20% (IQR 72.5 - 84) (P = 0.059).
Use of heart rate limiting medications such as beta-blockers was not significantly different between the VP burden groups (VP < 20%: 40.95% vs. VP ≥ 20%: 59.05%; P = 0.96). This was a similar trend for calcium channel blockers (VP < 20%: 38.6% vs. VP ≥ 20%: 61.4%; P = 0.73) and antiarrhythmic class 1 or class 3 medications (VP < 20%: 0%, 40.9% vs. VP ≥ 20%: 100%, 59.09%; P = 0.4, 0.99, respectively).
Admission ECG and echocardiography
All patients in VP < 20% (100%) had admission ECGs for review. One hundred and twenty-nine patients had admission ECG data available in VP ≥ 20% (83%). There was no significant difference between groups in terms of admission rhythm (median heart rate or QRS duration) (Table 2). The median PR interval in SR in VP ≥ 20% was found to be 200 ms, with half of that cohort demonstrating first-degree heart block > 200 ms. The median PR interval in VP < 20% was within the normal range at 190 ms.
 Click to view | Table 2. Admission ECG and Pre-Implant Echo Parameters |
In terms of echocardiographic parameters, 73 patients in VP < 20% (89%) had echocardiographic data available versus 115 patients in VP ≥ 20% (74%). No significant difference was observed between the two groups with respect to any of the chamber size, LV function or severity of valvular lesions examined.
PPM indication and implantation settings
When comparing implantation indications between both groups (Table 3), devices were inserted in VP < 20% and VP ≥ 20% for tachy-brady syndrome (18.3% vs. 20.8%), Mobitz 2 (9.8% vs. 10.4%), or transient high-grade AV block (14.6% vs. 16.9%) at relatively even distributions. VP < 20% comprised a greater proportion of symptomatic bradycardia patients (17.1% vs. 5.8%) and those with sustained CHB were significantly more likely to be in the VP ≥ 20% group (VP < 20%: n = 1 (1.2%) vs. VP ≥ 20%: n = 25 (16.2%); P = 0.002) (Table 3).
 Click to view | Table 3. Indications for PPM, Implantation Settings and VP% Burden Over 3 Years |
Additionally, we observed significant differences in terms of RV pacing mode switch algorithms (P < 0.001) (Table 3). VP < 20% utilized mode switch algorithms to a greater extent than VP ≥ 20% (67% vs. 19%). In contrast, VP ≥ 20% had more straightforward VVI or DDD modes at time of implantation (VP < 20%: 3.7%, 29.3% vs. VP ≥ 20%: 20.7%, 60.3%, respectively) (Table 3).
VP burden change over time
In the VP < 20% group, as shown in Figure 1, the mean pacing burden over 3 years in VP < 20% was 3% in year 1, 7% in year 2 and 11% in year 3. All individuals paced less than 20% VP in year 1. In the second year of follow-up, eight patients (9.75%) started pacing > 20% of the time, and in the third year, 21 patients (25.6%) paced > 20% of the time.
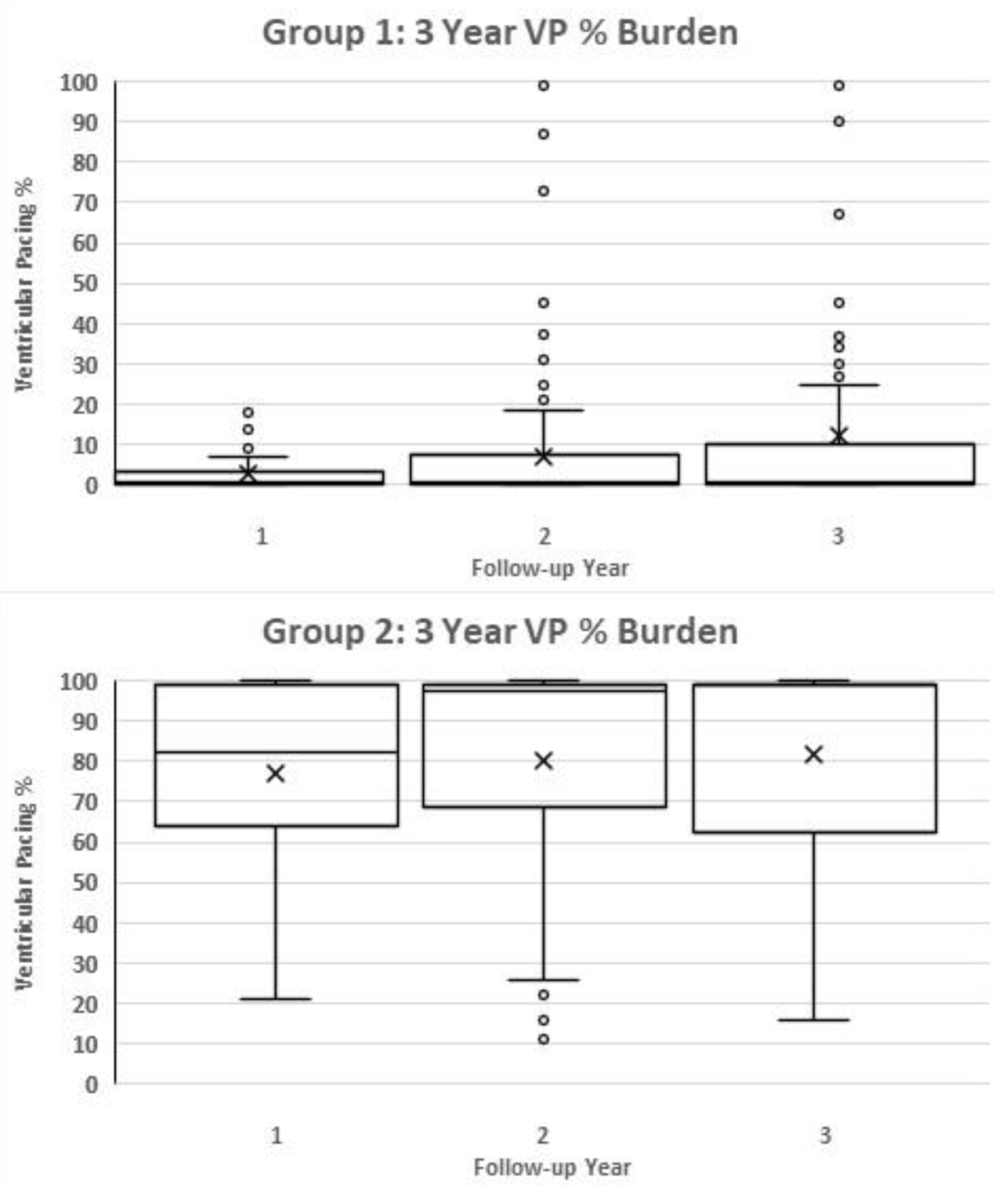 Click for large image | Figure 1. Box and whisker chart demonstrating the progression of VP% burden over 3 years in group 1 and group 2 individually. “X” represents the average value, while the box and the additional line contained within the box represent median and interquartile range. VP: ventricular pacing. |
In VP ≥ 20%, pacing burdens monitoring over 3 years demonstrated a mean of 76% in year 1, 80% in year 2, and 80.8% in year 3.
| Discussion | ▴Top |
PICM
Due to variations in defining PICM, the reported incidence within the literature can vary widely [2]. However, it has been demonstrated that the deleterious effects most commonly occur shortly after device implantation [15]. Patients who utilize their RV lead to a greater extent are more likely to develop this phenomenon which may involve clinical signs and symptoms of heart failure, LV systolic impairment, LV negative remodeling and dilation, mitral regurgitation and AF [16].
Poorer outcomes associated with RV pacing, as compared to intrinsic activation, reflect the interventricular dyssynchrony arising from abnormally late activation of the LV lateral wall which subsequently increases myocardial work and oxygen consumption [17, 18]. This may further lead to deteriorations in LVEF (< 50%) and PICM [19]. The negative deleterious effects of long-term RV pacing in heart failure patients were first described in the DAVID trial [20] and have since been expanded in both experimental and clinical studies.
Factors associated with high VP%
The leading risk factor for development of PICM has repeatedly reported to be a high RV pacing burden. This has been studied at gradually reducing thresholds, with data now demonstrating that a total burden of > 20% is an independent risk factor, therefore validating the cut-off utilized in the current study. To our knowledge, this is the first report to examine patient factors leading to a high RV pacing burden in bradyarrhythmia, with existing studies merely highlighting proposed theories of underlying ventricular rate.
Similar to previous reports examining independent predictors of PICM [15, 16], our results showed that persistent high-grade atrioventricular (AV) block was associated with a VP > 20%. Our patient cohort excluded ICDs and so the LVEFs of our patients are generally preserved. Notably, the incidence of PICM is strongly associated with an RV pacing burden > 20% in patients with CHB and preserved LVEF [17].
While previous studies have demonstrated higher RV pacing burden leading to worse clinical outcomes following PPM implantation [21-23], these findings remain much less refined in patients undergoing cardiac surgery, specifically CABG. Our study demonstrated a significant relationship between VP > 20% and prior CABG, which could be explained by a number of factors. While the cardiac conduction system is already disrupted in patients with valvular heart disease due to myocardial fibrosis and calcification, intraoperative tissue injury and local edema could also result in further cardiac system disturbances [24]. This may subsequently lead to significant AV block and bradyarrhythmias, requiring higher RV pacing burden and pacemaker “dependency” in the long term [25]. Even in the absence of coronary artery disease, changes in ventricular blood perfusion due to high non-physiological VP may lead to regional myocardial perfusion defects and widespread mechanical dyssynchrony.
Male sex was found to be an associated factor with high VP% in our study. Rather than being as a result of an independent sex-related physiological difference, we believe that this finding is likely secondary to differences in indication. It is well known that male sex is an independent risk factor for high-grade AV nodal disease [26].
Other factors known to increase the risk for PICM development include ischemic heart disease, likely due to higher pacing rates than the patient’s initial pre-implantation rate [21], age, and male gender. This is consistent with our results in which a significantly greater proportion of men were found to have a VP burden ≥ 20% at the time of analysis. Despite the fact that more men than women under the age of 80 years receive PPMs [27], male sex has been correlated with new onset LV systolic dysfunction following pacemaker implantation [28]. Male patients are also more likely to receive a pacemaker for AV block [29], which is independently associated with high pacing burden. Indeed, the incidence of PICM is strongly associated with an RV pacing burden > 20% in patients with CHB and preserved LVEF [1]. As described in our study, pacing > 20% was almost exclusively associated with CHB. Patients with transient high-grade AV block demonstrated a mixed picture over our 3 years of follow-up, but if followed for longer one may expect the transient AV block to become more sustained and pacing burden to increase. The indication of “transient high-grade AV block” comprised equal proportions of both groups; however, from an absolute number perspective, 26 of 38 patients (68%) categorized into this group paced > 20%. Whether patients with transient high-grade AV block should get a physiological device as first-line remains unclear. Progression of pacing burden between both groups over 3 years is displayed in Figure 1.
Notably, a wider baseline QRS complex has also been found to be significant in determining PICM, with an increased incidence of PICM with a paced QRS duration > 150 ms [1]. Albeit this finding has been identified in studies with small sample sizes and insufficient follow-up durations [14], these results lend itself to the clinical utility of electrocardiography as a non-invasive tool to identify QRS duration as a parameter of synchronous ventricular activation.
Further considerations: potential contributing factors
Our study found a trend towards significance when looking at patients with a background of AF with increased age at time of implant of device whether they were paced > 20% of the time. These factors would suggest worsened intrinsic conductive disease. Age at time of implant has been previously reported as a risk factor for incidence of PICM. Zhang et al found that for each year of life, the hazard ratio (HR) determined an increased risk of 6%, HR 1.06 (1.04 - 1.08, P < 0.001) [30]. They defined PICM by incident clinical heart failure, not via echocardiographic LVEF analyses. This risk was not replicated in all studies which used other definitions of PICM [31]. More recently, a meta-analysis by Somma et al of 57,993 patients found most contributory factors to be baseline LVEF, native QRS duration, RV pacing percentage and paced QRS duration [2]. Other risk factors did include male sex, history of myocardial infarction, or background of AF.
In our high VP% group, the median PR interval was qualified as first-degree heart block with 200 ms, in comparison the median PR interval in the low paced group was 190 ms, within the normal range. First-degree heart block may naturally progress to more significant AV block, and studies suggest that PR prolongation may be a risk marker for either progression to a more significant block over time, or the co-existence of transient high-grade AV block [32].
This phenomenon also raises the question of post-ventricular atrial refractory period (PVARP) optimization, where patients with first-degree AV block would be more likely to pace more frequently due to the operator settings on their device. First-degree AV block as a marker of more significant underlying conductive disease has been theorized as one of the reasons why patients from this cohort have worse responses to CRT than those without first-degree heart block [32, 33].
Clinical implications
Our findings suggest that patients who require a device insertion for CHB should strongly consider avoiding an RV only pacing device as their first-line pacing option, due to the high VP% burden and high risk of progressing to PICM, and these data can reasonably be extrapolated to any patient other than CHB indication who the treating physician is sure will be pacing dependant such as those post AV nodal ablation. Conversely, if a patient requires a device for sporadic and occasional symptomatic brady episodes without high-grade AV block, it is likely that an RV lead is acceptable given its low complication rate, less lab time and widespread availability.
Following our analyses, a challenge remains; for a remainder of patients, it is unclear as to whether they will be pacing dependant or not, or how quickly they will progress. We have identified several risk factors that are either significantly associated or trend towards significance in this single-center retrospective study which may in time comprise a weighted risk stratification tool or score.
Limitations
While the results of the present study are robust, there are a few limitations to consider. Firstly, as this is a retrospective chart review, an accurate record of indications for pacemaker insertion may have been complicated by the substantial overlap between tachy-brady syndrome, AF with pauses and symptomatic bradycardia. Our data are reliant on clinical record keeping.
Secondly, by excluding ICD implantation recipients, for the most part only patients with LVEF > 40% were captured. While our findings may not be generalizable to those who have severe LV dysfunction, the selection of our specific patient cohort was guided by current guidelines for his bundle pacing.
The study is likely to be statistically underpowered for comparisons due to the small sample size in some groups. Larger prospective studies with longer term follow-up are required to create more complex prediction models. Multiple statistical tests were applied to these data, and if a correction for this was applied, for example the Bonferroni correction, to reduce the risk of type 1 error, only the results showing associations with indications and PPM would remain. Another limitation is that this is a single-center study, and so potentially lacks generalizability of the results.
Conclusions
Unsurprisingly, sustained high-grade AV block was associated with an RV pacing burden > 20% over 3 years. Of note, this finding was not consistent in patients with transient episodes of high-grade AV. Careful consideration should be given to pacing lead implant technique in those who present with symptomatic non-sustained high-grade AV block as our data suggest these patients do not pace > 20% of the time when followed up over 3 years.
We found a significant association between high VP% and male sex in addition to previous CABG. Other factors to consider include prolonged PR interval, a history of AF or a more advanced age at time of implant. We also identified a significant association between the absence of proprietary ventricular mode switching algorithm use and increased VP%.
Learning points
RV pacing is still recommended as first-line bradycardia therapy in those who are expected to utilize VP occasionally, especially in the absence of other markers of marked underlying conductive disease.
Other than high-grade AV block, factors to consider as representing greater underlying conductive disease include male sex or previous CABG. Other important considerations include a history of AF, a prolonged PR interval or more advanced age at time of implant.
There is a distinct separation between groups in terms of pacing burden, with the majority either pacing very little (mean = 3%, 7% and 11% over 3 years, respectively) or very high (mean > 80% from year 1).
Acknowledgments
None to declare.
Financial Disclosure
No funding was attained for this study.
Conflict of Interest
We have no conflict of interest to declare.
Informed Consent
Not applicable.
Author Contributions
All authors contributed significantly in terms of data collection, analysis, writing, correction and final edits.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, Kanj M, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13(12):2272-2278.
doi pubmed - Somma V, Ha FJ, Palmer S, Mohamed U, Agarwal S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm. 2023;20(2):282-290.
doi pubmed - Abbas J, Zulqarnain M, Waqar F, Waqar Z, Malik J, Satti DI, Zaidi SMJ. Incidence and predictors of pacemaker-induced cardiomyopathy with right ventricular pacing: a systematic review. Expert Rev Cardiovasc Ther. 2022;20(4):267-273.
doi pubmed - Cronin EM, Jones P, Seth MC, Varma N. Right ventricular pacing increases risk of appropriate implantable cardioverter-defibrillator shocks asymmetrically: an analysis of the ALTITUDE database. Circ Arrhythm Electrophysiol. 2017;10(10):e004711.
doi pubmed - Jankelson L, Bordachar P, Strik M, Ploux S, Chinitz L. Reducing right ventricular pacing burden: algorithms, benefits, and risks. Europace. 2019;21(4):539-547.
doi pubmed - Loring Z, Giczewska A, Hofmann P, Chiswell K, Schlegel TT, Ugander M, Jackson KP, et al. Electrocardiographic parameters associated with pacemaker induced cardiomyopathy. J Electrocardiol. 2023;77:17-22.
doi pubmed - Abdelmohsen Sayed M, Abd El Fatah Badran H, Khaled S, Effat Fakhry E. Predictors of right ventricular pacing-induced left ventricular dysfunction in pacemaker recipients with preserved ejection fraction. Herzschrittmacherther Elektrophysiol. 2022;33(3):312-318.
doi pubmed - Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, Frankel DS. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11(9):1619-1625.
doi pubmed - Cho SW, Gwag HB, Hwang JK, Chun KJ, Park KM, On YK, Kim JS, et al. Clinical features, predictors, and long-term prognosis of pacing-induced cardiomyopathy. Eur J Heart Fail. 2019;21(5):643-651.
doi pubmed - Rickard J, Gold MR, Patel D, Wilkoff BL, Varma N, Sinha S, Albert C, et al. Long-term outcomes in nonprogressors to cardiac resynchronization therapy. Heart Rhythm. 2023;20(2):165-170.
doi pubmed - Ploux S, Eschalier R, Whinnett ZI, Lumens J, Derval N, Sacher F, Hocini M, et al. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm. 2015;12(4):782-791.
doi pubmed - Li Y, Yan L, Dai Y, Zhou Y, Sun Q, Chen R, Lin J, et al. Feasibility and efficacy of left bundle branch area pacing in patients indicated for cardiac resynchronization therapy. Europace. 2020;22(Suppl_2):ii54-ii60.
doi pubmed - Siranart N, Chokesuwattanaskul R, Prasitlumkum N, Huntrakul A, Phanthong T, Sowalertrat W, Navaravong L, et al. Reverse of left ventricular remodeling in heart failure patients with left bundle branch area pacing: Systematic review and meta-analysis. Pacing Clin Electrophysiol. 2023;46(6):459-466.
doi pubmed - Zheng R, Yao H, Lian L. His-Purkinje conduction system pacing for pacing-induced cardiomyopathy: a systematic literature review and meta-analysis. J Interv Card Electrophysiol. 2023;66(4):1005-1013.
doi pubmed - Dawood M, Elsharkawy E, Abdel-Hay MA, Nawar M. Predictors of pacing induced left ventricular dysfunction and cardiomyopathy assessed by three-dimensional echocardiography and speckle tracking strain. Egypt Heart J. 2021;73(1):10.
doi pubmed pmc - Link MS, Hellkamp AS, Estes NA, 3rd, Orav EJ, Ellenbogen KA, Ibrahim B, Greenspon A, et al. High incidence of pacemaker syndrome in patients with sinus node dysfunction treated with ventricular-based pacing in the Mode Selection Trial (MOST). J Am Coll Cardiol. 2004;43(11):2066-2071.
doi pubmed - Skalidis EI, Kochiadakis GE, Koukouraki SI, Chrysostomakis SI, Igoumenidis NE, Karkavitsas NS, Vardas PE. Myocardial perfusion in patients with permanent ventricular pacing and normal coronary arteries. J Am Coll Cardiol. 2001;37(1):124-129.
doi pubmed - Vernooy K, Dijkman B, Cheriex EC, Prinzen FW, Crijns HJ. Ventricular remodeling during long-term right ventricular pacing following His bundle ablation. Am J Cardiol. 2006;97(8):1223-1227.
doi pubmed - Kaye G, Ng JY, Ahmed S, Valencia D, Harrop D, Ng ACT. The Prevalence of Pacing-Induced Cardiomyopathy (PICM) in patients with long term right ventricular pacing - Is it a matter of definition? Heart Lung Circ. 2019;28(7):1027-1033.
doi pubmed - Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288(24):3115-3123.
doi pubmed - Merchant FM, Hoskins MH, Musat DL, Prillinger JB, Roberts GJ, Nabutovsky Y, Mittal S. Incidence and time course for developing heart failure with high-burden right ventricular pacing. Circ Cardiovasc Qual Outcomes. 2017;10(6):e003564.
doi pubmed - Zhang H, Zhou YJ, Zeng YJ. Prognostic factors of pacing-induced cardiomyopathy. Chin Med J (Engl). 2020;133(13):1533-1539.
doi pubmed pmc - Youssef A, Pfluecke C, Dawid M, Ibrahim K, Gunther M, Kolschmann S, Richter U, et al. The short term influence of right ventricular pacing burden on echocardiographic and spiroergometric parameters in patients with preserved left ventricular ejection fraction. BMC Cardiovasc Disord. 2022;22(1):23.
doi pubmed pmc - Raza SS, Li JM, John R, Chen LY, Tholakanahalli VN, Mbai M, Adabag AS. Long-term mortality and pacing outcomes of patients with permanent pacemaker implantation after cardiac surgery. Pacing Clin Electrophysiol. 2011;34(3):331-338.
doi pubmed - Steyers CM, 3rd, Khera R, Bhave P. Pacemaker dependency after cardiac surgery: a systematic review of current evidence. PLoS One. 2015;10(10):e0140340.
doi pubmed pmc - Shan R, Ning Y, Ma Y, Liu S, Wu J, Fan X, Lv J, et al. Prevalence and risk factors of atrioventricular block among 15 million Chinese health examination participants in 2018: a nation-wide cross-sectional study. BMC Cardiovasc Disord. 2021;21(1):289.
doi pubmed pmc - Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T, Gillis AM, et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. 2018;20(10):1565-1565ao.
doi pubmed - Cho EJ, Park SJ, Park KM, On YK, Kim JS. Paced QT interval as a risk factor for new-onset left ventricular systolic dysfunction and cardiac death after permanent pacemaker implantation. Int J Cardiol. 2016;203:158-163.
doi pubmed - Koo A, Stein A, Walsh R. Pacing-induced cardiomyopathy. Clin Pract Cases Emerg Med. 2017;1(4):362-364.
doi pubmed pmc - Zhang XH, Chen H, Siu CW, Yiu KH, Chan WS, Lee KL, Chan HW, et al. New-onset heart failure after permanent right ventricular apical pacing in patients with acquired high-grade atrioventricular block and normal left ventricular function. J Cardiovasc Electrophysiol. 2008;19(2):136-141.
doi pubmed - Lewalter T, Purerfellner H, Ungar A, Rieger G, Mangoni L, Duru F, investigators IXs. "First-degree AV block-a benign entity?" Insertable cardiac monitor in patients with 1st-degree AV block reveals presence or progression to higher grade block or bradycardia requiring pacemaker implant. J Interv Card Electrophysiol. 2018;52(3):303-306.
doi pubmed - Barold SS, Ilercil A, Leonelli F, Herweg B. First-degree atrioventricular block. Clinical manifestations, indications for pacing, pacemaker management & consequences during cardiac resynchronization. J Interv Card Electrophysiol. 2006;17(2):139-152.
doi pubmed - Januszkiewicz L, Vegh E, Borgquist R, Bose A, Sharma A, Orencole M, Mela T, et al. Prognostic implication of baseline PR interval in cardiac resynchronization therapy recipients. Heart Rhythm. 2015;12(11):2256-2262.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


