| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 1, February 2024, pages 18-28
Hemodynamic Response to Exercise Training in Heart Failure With Reduced Ejection Fraction Patients
Marine Kirscha, c, Marie-Christine Ilioub, Damien Vitielloa, c
aInstitut des Sciences du Sport Sante de Paris (I3SP), URP 3625, Universite Paris Cite, Paris 75015, France
bDepartment of Cardiac Rehabilitation and Secondary Prevention, Hopital Corentin Celton, APHP Centre, France
cCorresponding Author: Marine Kirsch and Damien Vitiello, Institut des Sciences du Sport Sante de Paris (I3SP), URP 3625, Universite Paris Cite, Paris 75015, Franceand
Manuscript submitted November 13, 2023, accepted December 27, 2023, published online February 28, 2024
Short title: Hemodynamic Response to Training in HFrEF Patients
doi: https://doi.org/10.14740/cr1591
| Abstract | ▴Top |
Background: Supervised exercise training decreases total and cardiac mortality and increases quality of life of heart failure with reduced ejection fraction (HFrEF) patients. However, response to training is variable from one patient to another and factors responsible for a positive response to training remain unclear. The aims of the study were to compare cardiac hemodynamic changes after an exercise training program in responders (R) versus non-responders (NR) HFrEF patients, and to compare different discriminators used to assess response to training.
Methods: Seventy-six HFrEF patients (86% males, 57 ± 12 years) completed an exercise training program for 4 weeks. Patients underwent cardiopulmonary exercise testing (CPET) on a cycle ergometer before and after training. Cardiac hemodynamics were measured by impedance cardiography during CPET. The R and NR groups were classified using the median change in peak oxygen uptake (V̇O2peak).
Results: There were statistically significant differences in V̇O2peak (+35% vs. -1%, P < 0.0001) and in peaks of ventilation (+30% vs. +2%, P < 0.0001), cardiac output (COpeak) (+25% vs. +4%, P < 0.01), systolic blood pressure (+12% vs. +2%, P < 0.05), diastolic blood pressure (+9% vs. +4%, P < 0.05) and heart rate (+8% vs. +1%, P < 0.01) between R and NR after the training program. V̇O2peak was the best discriminator between R and NR (receiver operating characteristic (ROC) area under the curve (AUC) = 0.83, P < 0.0001), followed by COpeak (ROC AUC = 0.77, P < 0.0001).
Conclusion: V̇O2peak is the best discriminator between HFrEF R and NR patients after the training program. Responders showed improvements in peak hemodynamic parameters. These results pave the way for other studies to determine how the individualization of exercise training programs and peak hemodynamic parameters potentially linked to a better positive response status.
Keywords: Heart failure; Cardiac rehabilitation; Exercise training; Hemodynamics; Individual response
| Introduction | ▴Top |
Exercise training is the cornerstone of secondary prevention and studies show the beneficial effects of exercise-based cardiac rehabilitation (CR) programs [1, 2], leading CR to be a strong recommendation from the European Society of Cardiology [3]. The beneficial effects of different modalities of exercise are well documented in non-pharmacological treatments of heart failure (HF). Aerobic training leads to central and peripheral adaptation, like an improvement of endothelial function [4], neuro-hormonal profile and exercise capacity [5], as well as an anti-inflammatory effect in chronic heart failure (CHF) patients [6]. High-intensity interval training induces improvements in cardiac output (CO), peak oxygen uptake (V̇O2peak), left ventricular ejection fraction (LVEF), endurance capacity and endothelial function and leads to a decrease in pro-brain natriuretic peptide [7]. Resistance training entails improvements in muscle structure, O2 convection, diffusion and utilization, decreases exercise intolerance [8] and induces favorable effects on the heart rate (HR) variability in CHF patients [9]. Finally, supervised exercise training leads to a decrease in total and cardiac mortality [10], increases quality of life and reduces hospital admissions of such patients [1].
Heterogeneity in exercise training responsiveness is multifactorial, ranging from genetics [11] to environmental components, or methodological factors (such as type, intensity or volume of exercise) [12], comorbidities or muscular strength [13]. A lack of improvement after training can be due to limiting circulatory factors [14], a chronotropic incompetence [15] or muscular deconditioning [16]. Response to training can therefore be highly variable from one patient to another [17], underlining the fact that clinical management should be more suitable to the specific needs of cardiac patients. Moreover, in 2021, a study showed that a physiological thresholds-based exercise training approach could lead to an acute physiological response which may manifest as more homogeneous chronic adaptations [12].
Guidelines confirm that exercise should be individually tailored, but a question remains unanswered: what would be the best way to individualize exercise training closer to patients’ needs and diseases characteristics? Change of baseline peak oxygen uptake (ΔV̇O2peak) after exercise training is a great predictor of mortality in cardiac patients and is frequently used to quantify improvements in patients’ exercise capacity [18, 19] and to predict long-term prognosis [5]. Moreover, ΔV̇O2peak is commonly used to classify patients’ response to training in different cardiac pathologies such as HF [20, 21] or coronary heart disease [22, 23]. However, we can find in the literature other ways to classify responders (R) and non-responders (NR) patients such as CO [14], oxygen convection, oxygen diffusion [21] or the relationship between minute ventilation and carbon dioxide production (V̇E/V̇CO2 slope) [15], suggesting that other variables could be useful to assess exercise training response. In this context, non-invasive measurements of cardiac hemodynamics parameters during exercise, such as impedance cardiography (ICG), have been validated to assess the determinants of exercise intolerance in cardiac patients [24-26], in order to identify responder patients, to evaluate cardiovascular adaptations to exercise training and to predict prognosis after the CR program [27]. For now, ICG has been mainly used to assess clinical management, predict clinical events, or estimate risks for cardiac patients.
Unlike invasive measurements, which are expensive, time-consuming, require expertise and could present risks for patients, ICG is safe to be used during exercise and has been shown to be accurate in cardiac patients [28]. ICG gives a valid measurement of CO, stroke volume (SV), systemic vascular resistance (SVR) during maximal exercise, allowing operators to assess physiological and hemodynamic changes in real time. Therefore, ICG is useful to optimize beneficial effects of training and to personalize exercise training prescription as part of CR by phenotyping cardiovascular adaptations [21, 29]. However, few studies have assessed the effects of exercise training on peak exercise cardiac hemodynamics in HFrEF patients. Despites these tries to assess the efficiency of exercise training program on response to training in HFrEF patients, little is known about determinants of a future positive response to exercise-based CR programs.
In this context, the aims of the present study were: 1) to compare cardiac hemodynamic changes after an exercise training program in R and NR patients with HFrEF; and 2) to compare the accuracy of different variables used to distinguish R from NR HFrEF patients.
| Materials and Methods | ▴Top |
Patients and study design
Between January 2015 and April 2018, 99 HFrEF patients referred for a structured exercise training program at the CR service of Corentin Celton Hospital were recruited in a randomized training intervention study (Fig. 1). The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Hospital Corentin Celton.
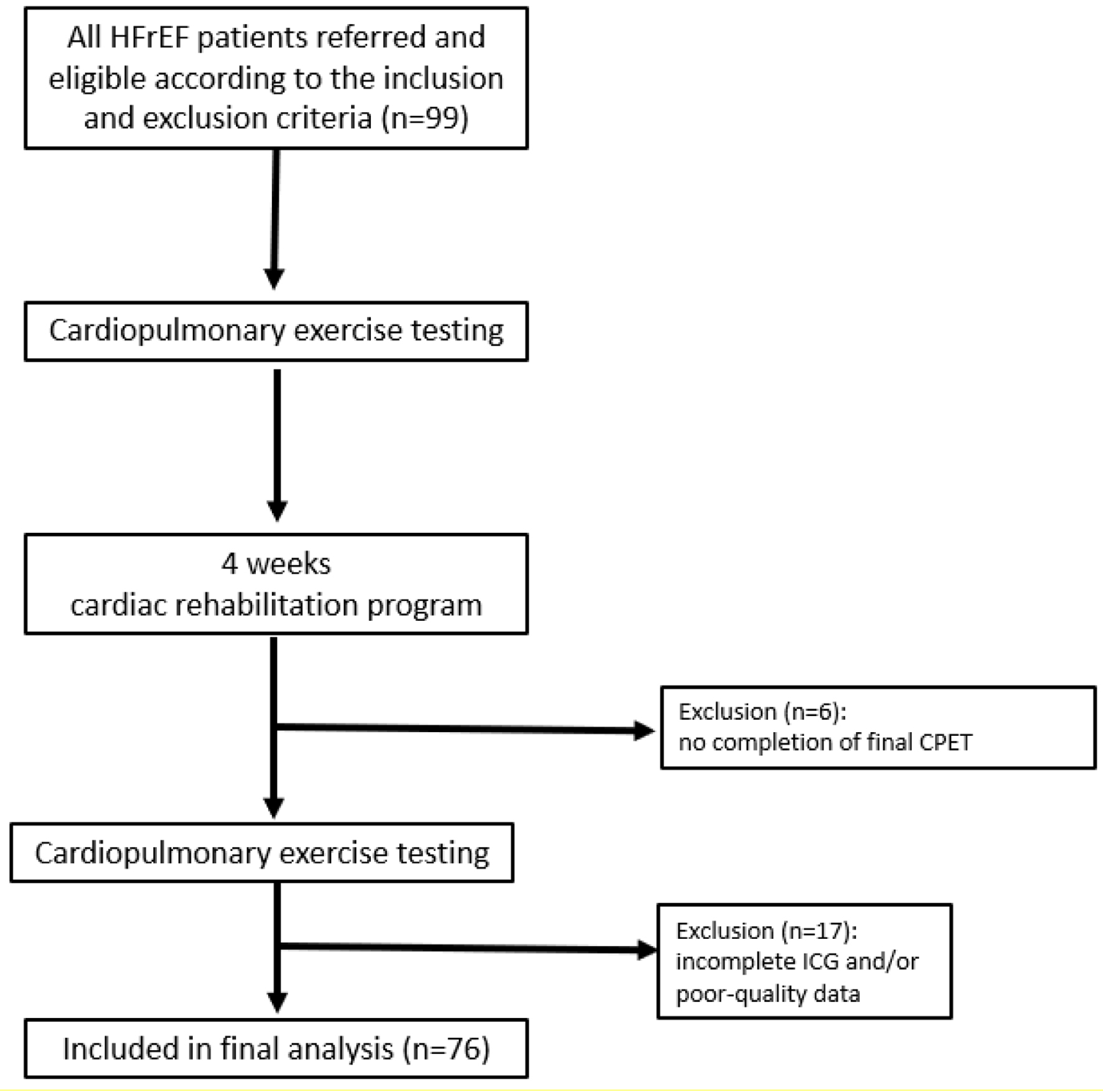 Click for large image | Figure 1. Flowchart of the study. CPET: cardiopulmonary exercise testing; HFrEF: heart failure with reduced ejection fraction; ICG: impedance cardiography. |
Patients were eligible if they were clinically stable, aged between 18 and 85 years; had a New York Heart Association (NYHA) functional class II to IIIb and an LVEF ≤ 40% assessed by echocardiography at rest. The exclusion criteria included valvular heart disease with surgical indication; acute HF < 15 days, myocardial infarction, coronary intervention and/or cardiac surgery < 4 weeks; severe pulmonary disease, patient unable to perform an exercise test or with a contraindication to cardiac rehabilitation according to French guidelines [30], resting systolic blood pressure (SBP) < 80 mm Hg or > 180 mm Hg, hemoglobin concentration < 9 g/dL, or poor echogenicity.
All patients underwent a physical examination, laboratory testing, including brain natriuretic peptide (BNP), creatinine, glomerular filtration rate using modification of diet in renal disease (MDRD) formula and hemoglobin, a standard rest Doppler echocardiography, and cardiopulmonary exercise testing (CPET) combined with an assessment of cardiac hemodynamic parameters before and after their CR program. The comprehensive CR program included exercise training, patient education, diet education, smoking, and psychosocial counseling.
Measurements
Clinical assessments (i.e., medical history, physical examination, and anthropometric measurements, cardiometabolic parameters, 12-h fasting blood analysis, CPET with gas exchange analysis, and ICG (PhysioFlow, Enduro, Manatec, France)) were performed at baseline and after completion of the exercise training program at the same time of day for each test.
CPET
The CPET was performed on a cycle ergometer (Sanabike 1.01, SDS Excellence version 2.8.2 Schiller, Switzerland), in a seated position. A ramp protocol of 10 W/min was used, until patients presented one of the following criteria: a respiratory exchange ratio > 1.05, inability to maintain cycling, exhaustion due to fatigue or clinical symptoms (e.g., dyspnea, electrocardiography (ECG), blood pressure abnormalities).
During the exercise tests, HR, SBP and diastolic blood pressure (DBP) were recorded at the beginning of each minute and at the maximal exercise intensity. Gas exchange parameters were measured breath by breath during testing, and then averaged every 15 s for minute ventilation (V̇E, L/min), O2 uptake (V̇O2, L/min) and CO2 production (V̇CO2, L/min) using an automated gas analyzer system (CPX Vyntus, Vyvaire Medical GmbH, Germany). The V̇O2peak and V̇E/V̇CO2 slope were also determined.
ICG
The ICG (PhysioFlow, Enduro model, Manatec, France) was used to measure cardiac hemodynamic changes during the CPET before and after the exercise training program. This non-invasive technique was found to be valid, accurate, and reproducible at rest and during exercise in healthy subjects and cardiac patients [31, 32]. Placement of electrodes was performed according to the user manual delivered with the device. CO (L/min), SBP (mm Hg), DPB (mm Hg), HR (bpm) and SVR (dyn.s/cm5) were measured with this device on a beat-to-beat basis and were then averaged every 15 s for data analysis. These variables were assessed to evaluate the impact of training on cardiac pre-load and post-load and global vascular compliance in our HFrEF patients.
Exercise training intervention
Exercise training intervention consisted in a combination of endurance, resistance and balance/stretching training sessions and was conducted by physiotherapists under the supervision of a cardiologist. The sessions of endurance training were performed on a cycle ergometer, 5 days/week (30 min/session) for 3 - 4 weeks. All sessions started with a 5-min warm-up phase and finished with 5-min a cool down phase. Continuous and/or interval training were alternating during a week. The initial intensity corresponded to the first ventilatory threshold (VT1) for the continuous type. Interval sessions were alternating, 1 min of exertion at 85-90% of the VO2peak interspaced with 3 - 4 min of recovery phase at intensity below the VT1. The intensity of training sessions was adapted every 2 or 3 sessions by increasing the workload by 5 - 10 W based on the Borg’s scale (12 - 14) [33].
The sessions of resistance training were conducted 3 days/week, 30 min per session. Patients underwent bodyweight strengthening of lower and upper limbs muscles.
Moreover, 30-min sessions of balance and stretching exercises were performed 5 days/week.
Classification of R and NR to training
The classification of R and NR patients was performed according to ΔV̇O2peak with training. The R and NR were differentiated based on the median change in peak V̇O2 (ΔV̇O2peak, mL/min/kg). Patients above the median change (ΔV̇O2peak > 2.2 mL/min/kg) were considered as R and patients under or equal to the median change (ΔV̇O2peak ≤ 2.2 mL/min/kg) were considered as NR.
Statistical analysis
Data are presented as means ± standard deviation for continuous variables and presented as frequencies and percentages for categorical variables. Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, Inc., La Jolla, CA, USA). After ensuring a normal distribution, baseline characteristics were compared between R and NR using a t-test and a Chi-square test for categorical variables. A two-way analysis of variance (ANOVA) models (group × time) with repeated measures for time were used to study the CPET and ICG parameters across time and between groups. Models with time, group, and group × time interaction as independent variables were used. The group × time interaction was the focus of the analysis as it tested the difference in the change (post - pre) between the two groups (R and NR). For changes in percentages (Δ post - pre) of peak hemodynamics, an ANOVA delta between groups was used. A P-value < 0.05 was considered statistically significant.
Concerning the training response discriminant, the receiver operating characteristic (ROC) area under the curve (AUC) was calculated for ΔV̇O2peak, ΔCO peak and ΔSVR peak. ROC curves indicate the true positive rate (sensitivity) of a test against its false positive rate (1 - specificity), whereas the AUC is a measure of the accuracy of the prediction test. An AUC between 0.5 and 0.7 was considered as a poor discrimination, between 0.7 and 0.8 as an acceptable discrimination and between 0.8 and 0.9 as an excellent discrimination.
| Results | ▴Top |
Baseline clinical characteristics
Among all HFrEF patients referred to the cardiac rehabilitation service between January 2015 and April 2018, 99 were eligible and 76 were finally included in the present study (women, n = 11 (14%), men, n = 65 (86%), 57 ± 12 years old). HFrEF patients presented hypertensive (17/76, 22%) and diabetic (7/76, 9%) profile. Patients were divided into two groups according to the median change in peak V̇O2 after training (ΔV̇O2peak > 2.2 mL/min/kg): 38 R and 38 NR. Baseline clinical characteristics of patients with CHD are presented in Table 1. There were no significant differences in baseline clinical characteristics, peri-procedural characteristics, cardiovascular risk factors and baseline medication.
 Click to view | Table 1. Baseline Characteristics of HFrEF Patients According to Their Training Response Status |
CPET and hemodynamics at rest and at peak exercise
Cardiac hemodynamic parameters at rest before and after the training program are presented in Table 2. There was a significant decrease in HR and a significant increase in SV after the training program (P > 0.05) for all HFrEF patients. Except for SBP and V̇E/V̇O2, all hemodynamic parameters were significantly altered by training (Table 2). Peak cardiac hemodynamic parameters before and after the training program for both R and NR groups are presented in Table 3. After the training program, there were no significant differences in V̇E/V̇O2 and SVR. There was a significant time and interaction effect (P < 0.0001) in V̇O2peak, which increased by 35% after training in R while remained the same in NR. There was also a group effect in V̇O2peak with higher values in R compared to NR (P < 0.01). The SBP and DBP increased in R with a significant time effect (P < 0.05) and without significant interaction effect. There was a significant time and interaction effect (P < 0.0001) in V̇E, which increased by 30% after training in R while remained the same in NR. HR and CO increased respectively by 8% and 25% in R with a significant time (P < 0.01) and interaction (P < 0.05) effect. HR and CO remained the same in NR after the training program. Both groups (NR and R) reduced V̇E/V̇CO2 after the training program, with a significant time effect (P < 0.05).
 Click to view | Table 2. Hemodynamic Parameters at Rest and at Peak |
 Click to view | Table 3. Hemodynamic Parameters at Peak for Responders and Non-Responders |
Changes at peak after the training program are illustrated in Figure 2. We observed significant changes in the R group in CO (+25%), DBP (+9%), HR (+8%), SBP (+12%), V̇E (+30%), and V̇O2peak (+35%), while changes in these hemodynamic parameters were not significant in the NR group, ranging from -1% to +4%.
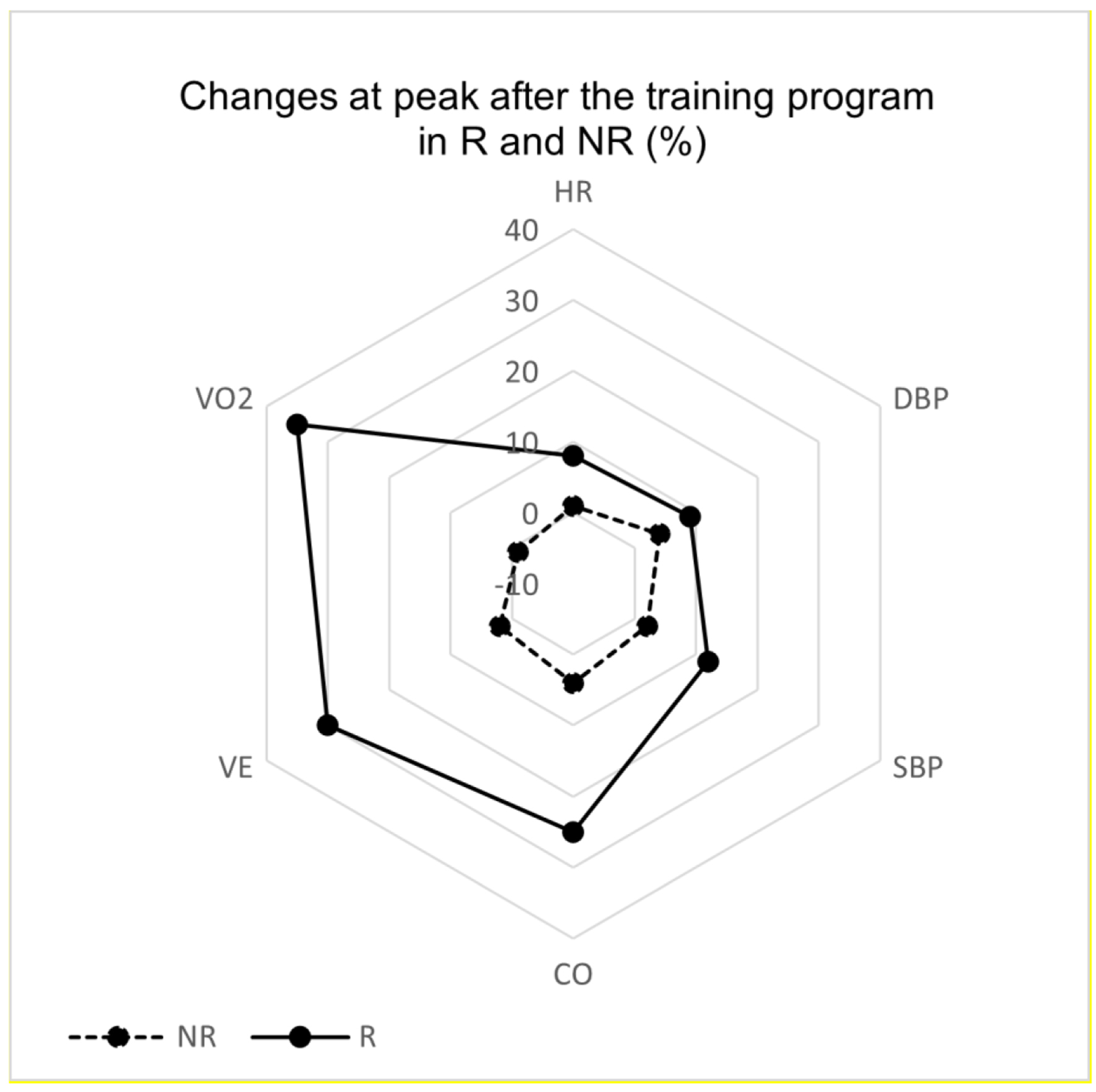 Click for large image | Figure 2. Radar changes in peak hemodynamic parameters in responders (R) and non-responders (NR). CO: cardiac output; DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure. |
ROC AUC
ROC analysis showed V̇O2peak to be an excellent discriminator between R and NR patients (AUC = 0.83, P < 0.0001; 95% confidence interval (CI): 0.7386 - 0.9180), and COpeak to be an acceptable discriminator (AUC = 0.77, P < 0.0001; 95% CI: 0.6653 - 0.8734). However, SVRpeak was found to be a poor discriminator (AUC = 0.67, P < 0.05; 95% CI: 0.5470 - 0.7910).
| Discussion | ▴Top |
To the best of our knowledge, this study is the first to non-invasively compare cardiac hemodynamic changes according to the training response status (defined by V̇O2peak change) in HFrEF patients. The main finding of the present study is that after training, peak CO, peak HR, peak V̇E, and peak SDP and DBP improved in the R group, while there were no significant changes concerning cardiac hemodynamics in the NR group. In addition, this study compared different discriminators to assess patients’ response to training. It appears that V̇O2peak is the best discriminator between R and NR patients, followed by COpeak as an acceptable discriminator, while SVRpeak was found to be a poor discriminator.
Differential impacts of training on hemodynamic parameters in HFrEF patients
The V̇O2peak improved by 35% in R, while it decreased by 1% in NR. Studies show that improvements in exercise capacity after a CR program are associated with an increase in V̇O2peak between 14% and 31% [34]. The change in V̇O2peak in our R group was slightly above the high gain average, which is an indicator of training effectiveness and implies a better prognosis in these patients [35]. In addition, the mean gain of V̇O2peak in the whole population (R and NR) was 2.3 mL/min/kg. This result is like those found in previous studies. Interestingly, a recent study showed that risks for readmissions for a cardiac event did not decrease with greater changes in V̇O2peak than 2 mL/min/kg [5]. The mean gain of all patients in this study leads to think that, regardless of the training response status, the CR program may decrease the risk of readmissions for our HFrEF patients. These results reinforce the necessity to propose a CR program to all HF patients to improve their physical condition and quality of life.
In addition, V̇E improved by 30% after the training program (46 vs. 60 L/min) in R. This result could be associated with the significant increase of SBPpeak and decrease of V̇E/V̇CO2, which are related to an improvement in ventilation efficiency. After the training program, SBPpeak increased by 12% in R (122 vs. 137 mm Hg) while remained unchanged in NR. In HFrEF patients, a high SBP is associated with better outcomes [36] and has been shown to be able to help improve existing prediction models. A high SBP has a protective survival effect, so our R patients may have a better clinical prognosis.
Moreover, both R and NR reduced V̇E/V̇CO2 after the training program (-2% in NR and -5% in R). The V̇E/V̇CO2 ratio has been suggested to be a strong parameter to assess ventilatory efficiency in HFrEF patients and the lowest V̇E/V̇CO2 ratio has been shown to be similar to the V̇E/V̇CO2 slope in predicting risk in such patients [37]. As a higher V̇E/V̇CO2 ratio has been associated with greater risks in HFrEF patients, our results suggest that the training program enhanced ventilatory efficiency and led to better outcomes in our patients.
Finally, HRpeak increased by 8% (108 ± 21 vs. 117 ± 25 bpm) and COpeak by 25% (8 ± 3 vs. 10 ± 3 L/min) in R after the training program. In NR, HRpeak increased by only 1% and COpeak by 4%. A meta-analysis in 2006 reported an increase of 22% in COpeak associated with a small increase in HRpeak of 2.5% after a training program [38]. These results are different from ours, which could be explained by a better improvement in cardiac performance in our population induced by a more adapted CR program. CHF patients depend on HR response to raise CO [39], already known as a predictor of good prognosis when increased after a training program. Interestingly, a study showed that a good chronotropic response to exercise is crucial for a positive response to training in terms of improvement in V̇O2peak [15], as CHF patients with chronotropic incompetence tend to have a lower V̇O2peak [40]. The same study also reported that HRpeak was a significant predictor of training response when analyzed by ROC curve.
It appears that a more adapted or personalized training program may induce more pronounced improvements of physical condition in HFrEF patients. Importantly, a more personalized training program may also improve the adherence of these patients and thus have higher beneficial effects [22].
The personalization of CR is therefore crucial to improve the quality of life of HF patients and one way to optimize this personalization may be the determination of other parameters able to finely discriminate response from non-response to exercise. Among these parameters, cardiac hemodynamic parameters may be of importance since disturbances in those have been thought to be responsible for exercise intolerance and major determinants of exercise capacity in HFrEF patients [41].
What is the best parameter to discriminate R from NR HFrEF patients to training?
In this study, ROC curve has been used to assess the overall discrimination performance of V̇O2peak, COpeak and SVRpeak. These two last parameters were chosen to be tested as they are related to circulatory mechanisms and therefore impact V̇O2peak. Results showed that V̇O2peak is the best discriminator to differentiate R from NR patients (Fig. 3). In the current literature, V̇O2peak is the most used way to classify patients’ response to exercise, as it allows to quantify improvements in exercise capacity and to predict long-term prognosis. The results of this study strengthen the fact that change in V̇O2peak is indeed a reliable way to assess the impact of a CR program.
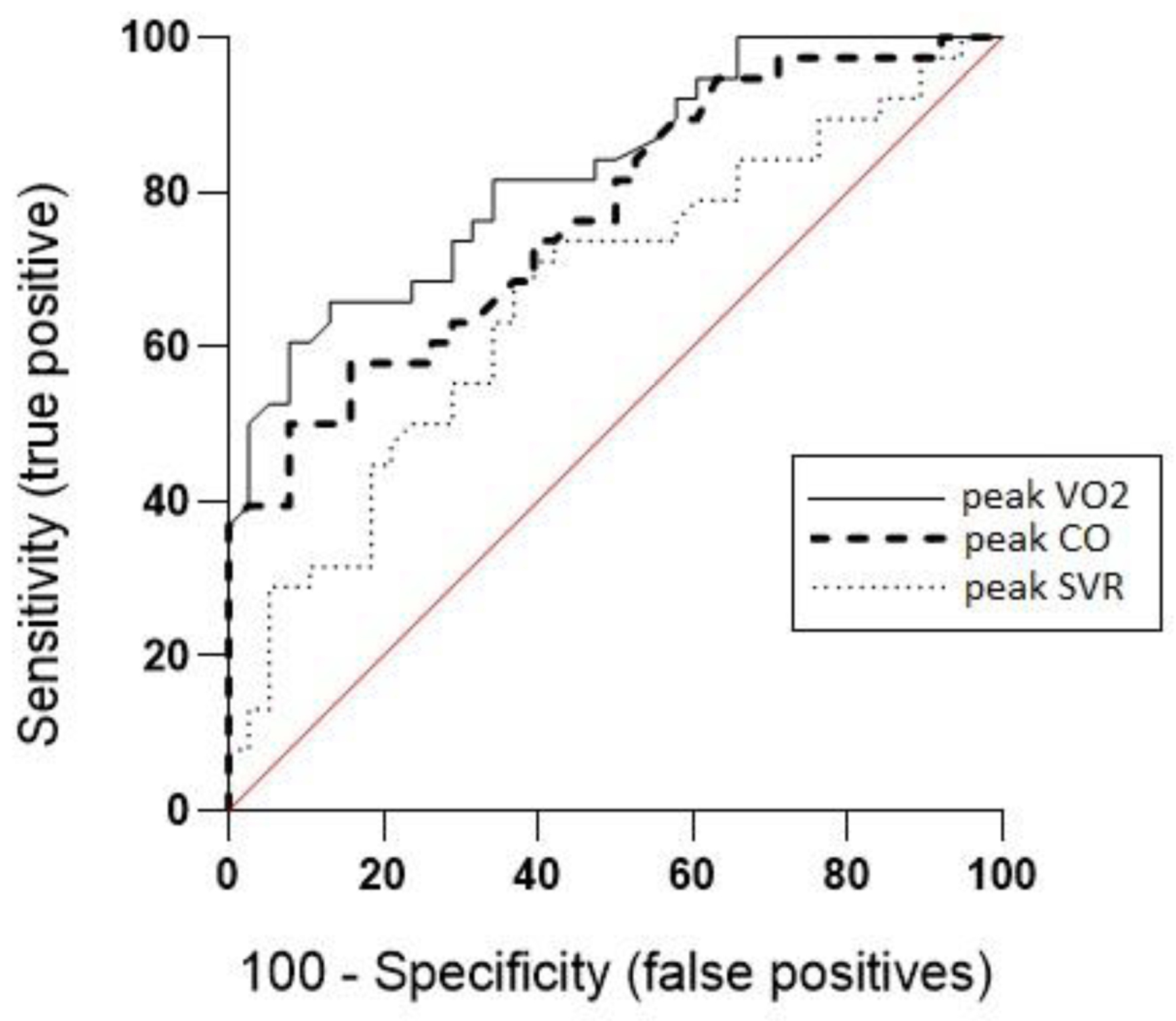 Click for large image | Figure 3. Receiver operating characteristic curves for discrimination between responder and non-responder HFrEF patients. CO: cardiac output; HFrEF: heart failure with reduced ejection fraction; SVR: systemic vascular resistance; V̇O2oxygen uptake. |
However, in this study COpeak has been shown to be an acceptable discriminator to assess response to training with an ROC AUC of 0.67, suggesting that this parameter could be added to the commonly used V̇O2peak discrimination, in order to be more precise in assessing the response to exercise. In 2021, a study used this parameter to differentiate R from NR patients according to the different steps of oxygen transport [21]. In previous studies, patients with an increased CO after training were reported to be R [14] and in 1996, a study showed that the possibility to increase blood flow during exercise, that to say a better CO, was a sine qua non to a positive response to training [14]. COpeak consequently appears to be a potential alternative way to assess response to training and to classify patients according to their status response.
Due to its relationship with CO during exercise, the V̇E/ V̇CO2 slope is used to assess HF severity [42] and therefore could also be used as a discriminator between R and NR patients. As a matter of fact, one study used V̇E/V̇CO2 slope as a discriminator, as R were defined by an improvement in V̇E/V̇CO2 slope by more than 5% [15].
To be more and more specific in predicting response to exercise and classifying R and NR to exercise, more parameters could be tested with ROC analysis such as other hemodynamic, echocardiographic or even muscular parameters. Using more than one parameter to assess response to exercise could strengthen the classification of R and potentially lead to an early identification of a non-response status, increasing benefits of CR program.
Limitations
The major limitation of the present study is the relatively small size of the population.
Furthermore, even though this method is commonly used in the literature, the choice of median change in V̇O2peak as a discriminator for training response remains an arbitrary one.
Additionally, using a non-invasive method has numerous advantages, especially for patients, but is not yet a standardized clinical method for measuring hemodynamic parameters. Invasive methods are still widely used.
Finally, the female gender is underrepresented, which can impact the results due to physiological gender-related differences also observed in exercise response.
Conclusion
R HFrEF patients showed improvements in peak hemodynamic parameters compared to their NR peers. In addition, it has been demonstrated that V̇O2peak was the best discriminator between R and NR patients.
The present study emphasizes the association between personalization of exercise training programs and peak hemodynamic parameters linked to a positive response status. The determination of new parameters able to better discriminate R to NR HF patients is needed to better personalized future training programs and better improve their quality of life.
Acknowledgments
None to declare.
Financial Disclosure
The present article was supported by the BRIO funding of the Universite Paris Cite.
Conflict of Interest
None to declare.
Informed Consent
All patients provided written informed consent to the experimental protocol before participating in the study.
Author Contributions
MCI and DV: conception and design of the study; MCI: acquisition of data; MK and DV: analysis and interpretation of data, drafting of the manuscript or revising it critically for important intellectual content; MCI, MK, and DV: final approval of the submitted version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AUC: area under the curve; CHF: chronic heart failure; CO: cardiac output; COpeak: peak cardiac output; CPET: cardiopulmonary exercise testing; CR: cardiac rehabilitation; DBP: diastolic blood pressure; HF: heart failure; HFrEF: heart failure with reduced ejection fraction; HR: heart rate; ICG: impedance cardiography; LVEF: left ventricular ejection fraction; NR: non-responders; R: responders; ROC: receiver operating characteristics; SBP: systolic blood pressure; SV: stroke volume; SVR: systemic vascular resistance; V̇E: ventilation; V̇E/V̇O2: minute ventilation/oxygen production; V̇E/V̇CO2: minute ventilation/carbon dioxide production; V̇O2: oxygen uptake; V̇O2peak: peak oxygen uptake; ΔV̇O2peak: change of peak oxygen uptake between pre- and post-training; VT1: first ventilatory threshold
| References | ▴Top |
- Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1(1):CD003331.
doi pubmed pmc - Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(20):2675-2682.
doi pubmed - Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, Collet JP, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42(1):17-96.
doi pubmed - Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93(2):210-214.
doi pubmed - Mikkelsen N, Cadarso-Suarez C, Lado-Baleato O, Diaz-Louzao C, Gil CP, Reeh J, Rasmusen H, et al. Improvement in VO(2peak) predicts readmissions for cardiovascular disease and mortality in patients undergoing cardiac rehabilitation. Eur J Prev Cardiol. 2020;27(8):811-819.
doi pubmed - Laoutaris ID. The 'aerobic/resistance/inspiratory muscle training hypothesis in heart failure'. Eur J Prev Cardiol. 2018;25(12):1257-1262.
doi pubmed - Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086-3094.
doi pubmed - Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58(13):1353-1362.
doi pubmed pmc - Saeidi M, Ravanbod R, Pourgharib Shahi MH, Navid H, Goosheh B, Baradaran A, Torkaman G. The Acute Effects of 2 Different Intensities of Resistance Exercise on Autonomic Function in Heart Failure Patients: A Randomized Controlled Trial. Anatol J Cardiol. 2023;27(5):266-273.
doi pubmed pmc - Ekblom O, Cider A, Hambraeus K, Back M, Leosdottir M, Lonn A, Borjesson M. Participation in exercise-based cardiac rehabilitation is related to reduced total mortality in both men and women: results from the SWEDEHEART registry. Eur J Prev Cardiol. 2022;29(3):485-492.
doi pubmed - Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985). 1999;87(3):1003-1008.
doi pubmed - Meyler S, Bottoms L, Muniz-Pumares D. Biological and methodological factors affecting V̇O2max response variability to endurance training and the influence of exercise intensity prescription. Exp Physiol. 2021;106(7):1410-1424.
doi pubmed - Savage PD, Antkowiak M, Ades PA. Failure to improve cardiopulmonary fitness in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2009;29(5):284-291.
doi pubmed - Wilson JR, Groves J, Rayos G. Circulatory status and response to cardiac rehabilitation in patients with heart failure. Circulation. 1996;94(7):1567-1572.
doi pubmed - Schmid JP, Zurek M, Saner H. Chronotropic incompetence predicts impaired response to exercise training in heart failure patients with sinus rhythm. Eur J Prev Cardiol. 2013;20(4):585-592.
doi pubmed - Kemps HM, Schep G, de Vries WR, Schmikli SL, Zonderland ML, Thijssen EJ, Wijn PF, et al. Predicting effects of exercise training in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2008;102(8):1073-1078.
doi pubmed - Ross R, Goodpaster BH, Koch LG, Sarzynski MA, Kohrt WM, Johannsen NM, Skinner JS, et al. Precision exercise medicine: understanding exercise response variability. Br J Sports Med. 2019;53(18):1141-1153.
doi pubmed pmc - Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024-2035.
doi pubmed - Mapelli M, Salvioni E, Mattavelli I, Vignati C, Galotta A, Magri D, Apostolo A, et al. Cardiopulmonary exercise testing and heart failure: a tale born from oxygen uptake. Eur Heart J Suppl. 2023;25(Suppl C):C319-C325.
doi pubmed pmc - Bakker EA, Snoek JA, Meindersma EP, Hopman MTE, Bellersen L, Verbeek ALM, Thijssen DHJ, et al. Absence of Fitness Improvement Is Associated with Outcomes in Heart Failure Patients. Med Sci Sports Exerc. 2018;50(2):196-203.
doi pubmed - Legendre A, Moatemri F, Kovalska O, Balice-Pasquinelli M, Blanchard JC, Lamar-Tanguy A, Ledru F, et al. Responses to exercise training in patients with heart failure. Analysis by oxygen transport steps. Int J Cardiol. 2021;330:120-127.
doi pubmed - De Schutter A, Kachur S, Lavie CJ, Menezes A, Shum KK, Bangalore S, Arena R, et al. Cardiac rehabilitation fitness changes and subsequent survival. Eur Heart J Qual Care Clin Outcomes. 2018;4(3):173-179.
doi pubmed - Trachsel LD, Boidin M, Henri C, Fortier A, Lalonge J, Juneau M, Nigam A, et al. Women and men with coronary heart disease respond similarly to different aerobic exercise training modalities: a pooled analysis of prospective randomized trials. Appl Physiol Nutr Metab. 2021;46(5):417-425.
doi pubmed - Krzesinski P, Gielerak G, Kowal J. [Impedance cardiography - a modern tool for monitoring therapy of cardiovascular diseases]. Kardiol Pol. 2009;67(1):65-71.
pubmed - Lasater M, Von Rueden KT. Outpatient cardiovascular management utilizing impedance cardiography. AACN Clin Issues. 2003;14(2):240-250.
doi pubmed - Kemps HM, Thijssen EJ, Schep G, Sleutjes BT, De Vries WR, Hoogeveen AR, Wijn PF, et al. Evaluation of two methods for continuous cardiac output assessment during exercise in chronic heart failure patients. J Appl Physiol (1985). 2008;105(6):1822-1829.
doi pubmed - Packer M, Abraham WT, Mehra MR, Yancy CW, Lawless CE, Mitchell JE, Smart FW, et al. Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol. 2006;47(11):2245-2252.
doi pubmed - Lang CC, Karlin P, Haythe J, Tsao L, Mancini DM. Ease of noninvasive measurement of cardiac output coupled with peak VO2 determination at rest and during exercise in patients with heart failure. Am J Cardiol. 2007;99(3):404-405.
doi pubmed - Lepretre PM, Poty A, Porcher T, Hermel F, Germain AL, Krim F, et al. Changes in exercise capacity of frailest patients with heart failure treated with standard exercise recommandations versus stroke volume response to exercise: a pilot study. Eur Heart J. 2018;39(suppl_1):ehy566. P6061.
- Pavy B, Iliou MC, Verges-Patois B, Brion R, Monpere C, Carre F, Aeberhard P, et al. French Society of Cardiology guidelines for cardiac rehabilitation in adults. Arch Cardiovasc Dis. 2012;105(5):309-328.
doi pubmed - Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793-801.
doi pubmed - Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106(6):666-671.
doi pubmed - Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
pubmed - Experience from controlled trials of physical training in chronic heart failure. Protocol and patient factors in effectiveness in the improvement in exercise tolerance. European Heart Failure Training Group. Eur Heart J. 1998;19(3):466-475.
doi pubmed - Tabet JY, Meurin P, Beauvais F, Weber H, Renaud N, Thabut G, Cohen-Solal A, et al. Absence of exercise capacity improvement after exercise training program: a strong prognostic factor in patients with chronic heart failure. Circ Heart Fail. 2008;1(4):220-226.
doi pubmed - Ather S, Chan W, Chillar A, Aguilar D, Pritchett AM, Ramasubbu K, Wehrens XH, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J. 2011;161(3):567-573.
doi pubmed pmc - Myers J, Arena R, Oliveira RB, Bensimhon D, Hsu L, Chase P, Guazzi M, et al. The lowest VE/VCO2 ratio during exercise as a predictor of outcomes in patients with heart failure. J Card Fail. 2009;15(9):756-762.
doi pubmed pmc - van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8(8):841-850.
doi pubmed - Higginbotham MB, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51(1):52-60.
doi pubmed - Clark AL, Coats AJ. Chronotropic incompetence in chronic heart failure. Int J Cardiol. 1995;49(3):225-231.
doi pubmed - Myers J, Froelicher VF. Hemodynamic determinants of exercise capacity in chronic heart failure. Ann Intern Med. 1991;115(5):377-386.
doi pubmed - Paolillo S, Veglia F, Salvioni E, Corra U, Piepoli M, Lagioia R, Limongelli G, et al. Heart failure prognosis over time: how the prognostic role of oxygen consumption and ventilatory efficiency during exercise has changed in the last 20 years. Eur J Heart Fail. 2019;21(2):208-217.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


