| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 1, February 2023, pages 45-53
Is T-Wave Alternans a Repolarization Abnormality Marker in COVID-19? An Investigation on the Potentialities of Portable Electrocardiogram Device
Alexander Edo Tondasa, b , Dian Andina Munawarc, d
, Ilaria Marcantonie
, Iche Andriyani Libertyf
, Rido Mulawarmanf
, Muhammad Hadif
, Monica Trifitrianaf
, Taufik Indrajayag, Muhammad Yaminh
, Irfannuddin Irfannuddinf, i
, Laura Burattinie
aDepartment of Cardiology and Vascular Medicine, Mohammad Hoesin General Hospital, Palembang, Sumatera Selatan, Indonesia
bBiomedicine Doctoral Program, Faculty of Medicine, Universitas Sriwijaya, Palembang, Indonesia
cDepartment of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
dDepartment of Cardiology, Lyell Mcewin Hospital, School of Medicine, The University of Adelaide, Australia
eDepartment of Information Engineering, Universita Politecnica delle Marche, Ancona, Italy
fFaculty of Medicine, Universitas Sriwijaya Palembang, Indonesia
gCardiovascular Division, Department of Internal Medicine, Faculty of Medicine, Universitas Sriwijaya, Palembang, Indonesia
hCardiovascular Division, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
iCorresponding Author: Irfannuddin, Faculty of Medicine, Universitas Sriwijaya Palembang, Indonesia
Manuscript submitted December 9, 2022, accepted December 27, 2022, published online February 25, 2023
Short title: TWA in COVID-19
doi: https://doi.org/10.14740/cr1458
| Abstract | ▴Top |
Background: Cardiac arrhythmias are significantly associated with poor outcomes in coronavirus disease 2019 (COVID-19) patients. Microvolt T-wave alternans (TWA) can be automatically quantified and has been recognized as a representation of repolarization heterogeneity and linked to arrhythmogenesis in various cardiovascular diseases. This study aimed to explore the correlation between microvolt TWA and COVID-19 pathology.
Methods: Patients suspected of COVID-19 in Mohammad Hoesin General Hospital were consecutively evaluated using Alivecor® Kardiamobile 6L™ portable electrocardiogram (ECG) device. Severe COVID-19 patients or those who are unable to cooperate in active ECG self-recording were excluded from the study. TWA was detected and its amplitude was quantified using the novel enhanced adaptive match filter (EAMF) method.
Results: A total of 175 patients, 114 COVID-19 patients (polymerase chain reaction (PCR)-positive group), and 61 non-COVID-19 patients (PCR-negative group) were enrolled in the study. PCR-positive group was subdivided according to the severity of COVID-19 pathology into mild and moderate severity subgroups. Baseline TWA levels were similar between both groups during admission (42.47 ± 26.52 µV vs. 44.72 ± 38.21 µV), but higher TWA levels were observed during discharge in the PCR-positive compared to the PCR-negative group (53.45 ± 34.42 µV vs. 25.15 ± 17.64 µV, P = 0.03). The correlation between PCR-positive result in COVID-19 and TWA value was significant, after adjustment of other confounding variables (R2 = 0.081, P = 0.030). There was no significant difference in TWA levels between mild and moderate severity subgroups in patients with COVID-19, both during admission (44.29 ± 27.14 µV vs. 36.75 ± 24.46 µV, P = 0.34) and discharge (49.47 ± 33.62 µV vs. 61.09 ± 35.99 µV, P = 0.33).
Conclusions: Higher TWA values can be observed on follow-up ECG obtained during discharge in the PCR-positive COVID-19 patients.
Keywords: T-wave alternans; COVID-19; Cardiac repolarization; ECG device
| Introduction | ▴Top |
Coronavirus disease 2019 (COVID-19) is an ongoing global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To date, there have been more than six million total cases of COVID-19 in Indonesia with > 150,000 deaths reported [1, 2]. It has been shown that COVID-19 effects are not only limited to the lungs but also systemic consequences including the cardiovascular system and beyond [3-6]. Several studies have reported that the occurrence of cardiac arrhythmias was significantly associated with an increased risk of mortality and morbidity in COVID-19 [7, 8]. Furthermore, cardiac arrhythmias, such as sinus tachycardia and atrial fibrillation, may manifest and persist as part of the long COVID-19 syndrome, up to 12 months after the acute infection of the SARS-CoV-2 virus [3]. Dysrhythmias in COVID-19 are considered an interaction between direct tissue effects of the virus and systemic illness, and several studies have highlighted the exclusive association of heart repolarization abnormalities with COVID-19 pathomechanism, aside from administered drug effects [4, 7-10]. Although COVID-19 also affects atrial repolarization, it seems that ventricular repolarization heterogeneity has a more obvious correlation with disease severity [5, 11, 12]. Repolarization parameters measured with a surface electrocardiogram (ECG) at admission may be able to predict 30-day and 1-year outcomes in COVID-19 patients, thus emphasizing their prognostic role [13, 14]. Nevertheless, the optimal measurement and interpretation for manual ECG acquisition for some of these variables are still unknown and carry the risk of interobserver variability [15].
For the last few decades, the microvolt T-wave alternans (TWA) has been recognized and linked to arrhythmogenesis as a representation of ventricular repolarization heterogeneity, associated with both inducible and spontaneous ventricular tachyarrhythmias linked to fatality in the setting of both cardiovascular or non-cardiovascular diseases. It is characterized by a beat-to-beat fluctuation in the morphology and amplitude of the T-wave. Traditionally, TWA values can be obtained using special algorithms embedded to exercise testing or ambulatory electrocardiograms (AECG) recording apparatus, such as the modified moving average (MMA) or spectral method [16, 17].
During the pandemic, efforts are made to minimize physical contact and the risk of infection transmission in routine medical examinations [18, 19]. In this context, the use of portable and wearable digital ECG devices (also easily manageable by the patients themselves) may represent a solution. The introduction of such devices for the detection and management of arrhythmia has enabled new ways to analyze TWA in various clinical conditions, including COVID-19 [18, 20]. Here we conducted the first study aimed at investigating TWA as a repolarization abnormality marker in COVID-19 patients whose short ECG recordings were acquired using the Kardiamobile™ 6L portable device and analyzed using the enhanced adaptive matched filter (EAMF) method [21].
| Materials and Methods | ▴Top |
Study population and design
This study was a single-center, prospective observational study, performed at Mohammad Hoesin General Hospital, Palembang, Indonesia. All patients admitted with clinical suspicion of COVID-19 from July 2020 to November 2021 were consecutively enrolled. Patients were excluded if they had missing PCR results or severe COVID-19 symptoms, or if they were not able to follow instructions for portable ECG device setup. The standard methodology of this study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline [22].
Ethical issues
The current study protocol was approved by the Institutional Committee of Research and Ethics of Mohammad Hoesin General Hospital (Ref: No. 28/kepkrsmh/2020).
Data collection
The demographic characteristics of the study subjects, including characteristics, comorbidities, and medication history, were recorded from medical records. The diagnosis of COVID-19 was confirmed using real-time polymerase chain reaction (RT-PCR) nasopharyngeal swabs (Sansure MA-6000 RT PCR) with cutoff cycle threshold (CT) value of > 40. If the initial PCR test result was negative on admission day, the patient would be transferred to isolation rooms and treated while waiting for a second swab test on the next day to see if the patient turned out to be PCR-positive. The degree of severity of confirmed COVID-19 cases was categorized as mild, moderate, and severe, in concordance with the American College of Emergency Physicians (ACEP) guidelines [23]. Mild illness was defined as the presence of any various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell), without shortness of breath, dyspnea, or abnormal chest imaging. Moderate illness was defined as the evidence of lower respiratory tract disease during clinical assessment or imaging or having an oxygen saturation (SpO2) of ≥ 94% on room air at sea level. Severe illness was diagnosed if the following signs occurred: SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, respiratory frequency > 30 breaths per minute, or > 50% lung infiltrates on X-ray [24].
ECG signals were recorded on admission and discharge using a portable ECG device (Kardiamobile™ 6L (AliveCor®, Mountainview, California, USA)). Kardiamobile™ 6L (300 Hz sampling frequency and 14-bit resolution) can yield recordings from two primary leads. Data were extracted from lead II, which represents the direction of the cardiac electrical vector. Each patient was given a piece of detailed information on how to perform acquisition using the Kardiamobile™ 6L recorder. ECG acquisition duration was set to 3 min and recorded data were wirelessly transmitted to a preinstalled smartphone-based application (Alivecor® Kardia) to be simultaneously saved locally and into the cloud system.
EAMF for microvolt TWA measurement
TWA was measured only in ECG recordings characterized by a mean heart rate between 50 and 130 bpm, and heart-rate variability lower than 30% of the mean heart rate. Specifically, TWA was measured by application of the EAMF method to ECG windows containing 64 consecutive heartbeats recursively extracted from the original tracing. Each ECG window was preprocessed for baseline removal and T-wave localization (from J point to T endpoint) and then tested to verify its suitability to be further analyzed for TWA measurement [21]. An ECG window was considered suitable if the number of ectopic or artifact-affected heartbeats did not exceed five beats and RR-interval standard deviation did not overcome 10% of the mean RR interval; otherwise, it was discarded. Suitable ECG windows were enhanced by setting to baseline all ECG waves but the T wave, and filtered with a very narrow (0.12 Hz) pass band filter (sixth-order bidirectional Butterworth filter) with the band centered at half heart rate, by definition equal to the alternans frequency. Thus, the signal at the output of the filter was a pseudo-sinusoidal signal, the amplitude of which provided a measurement of the TWA (µV) phenomenon characterizing the ECG window. If for an ECG tracing there is more than one suitable ECG window, and thus more than one TWA value, these are averaged to obtain a single TWA measure for each ECG tracing [21].
Statistical analysis
The statistical analysis was done using STATA version 15 (College Station, Texas, USA). Numerical data were described as mean and standard deviations (SDs), while categorical data were displayed as proportional numbers (%). Data distribution was tested using one-sample Kolmogorov-Smirnov test. Continuous variables with normal distribution were analyzed by an independent t-test. The Chi-square test was used to compare categorical variables. Z-test was implemented to analyze the significance of TWA abnormality categories during admission and discharge. Analysis of the correlation between PCR result and TWA was determined by multivariate linear regression after adjusting for comorbidities, electrolytes, and medications during hospitalization. Statistical significance for all tests was set to 0.05.
| Results | ▴Top |
Patients’ characteristics
During the study period, there were 207 patients admitted with a clinical diagnosis of COVID-19. Of these, 11 patients were excluded due to severe COVID-19 and/or uncooperative, while 22 patients did not give consent to participate in the study. As a result, 175 patients were eligible to perform Kardia measurement, with 114 patients categorized into the PCR-positive group and 61 patients in the PCR-negative control group according to their swab test results. Overall, 76 patients and 34 patients were analyzed in PCR-positive and PCR-negative groups, respectively. In total, 86 admission TWA data and 58 discharge TWA were acquired from available portable ECG data signals, while the rest missed either admission or discharge ECG recording (Fig. 1).
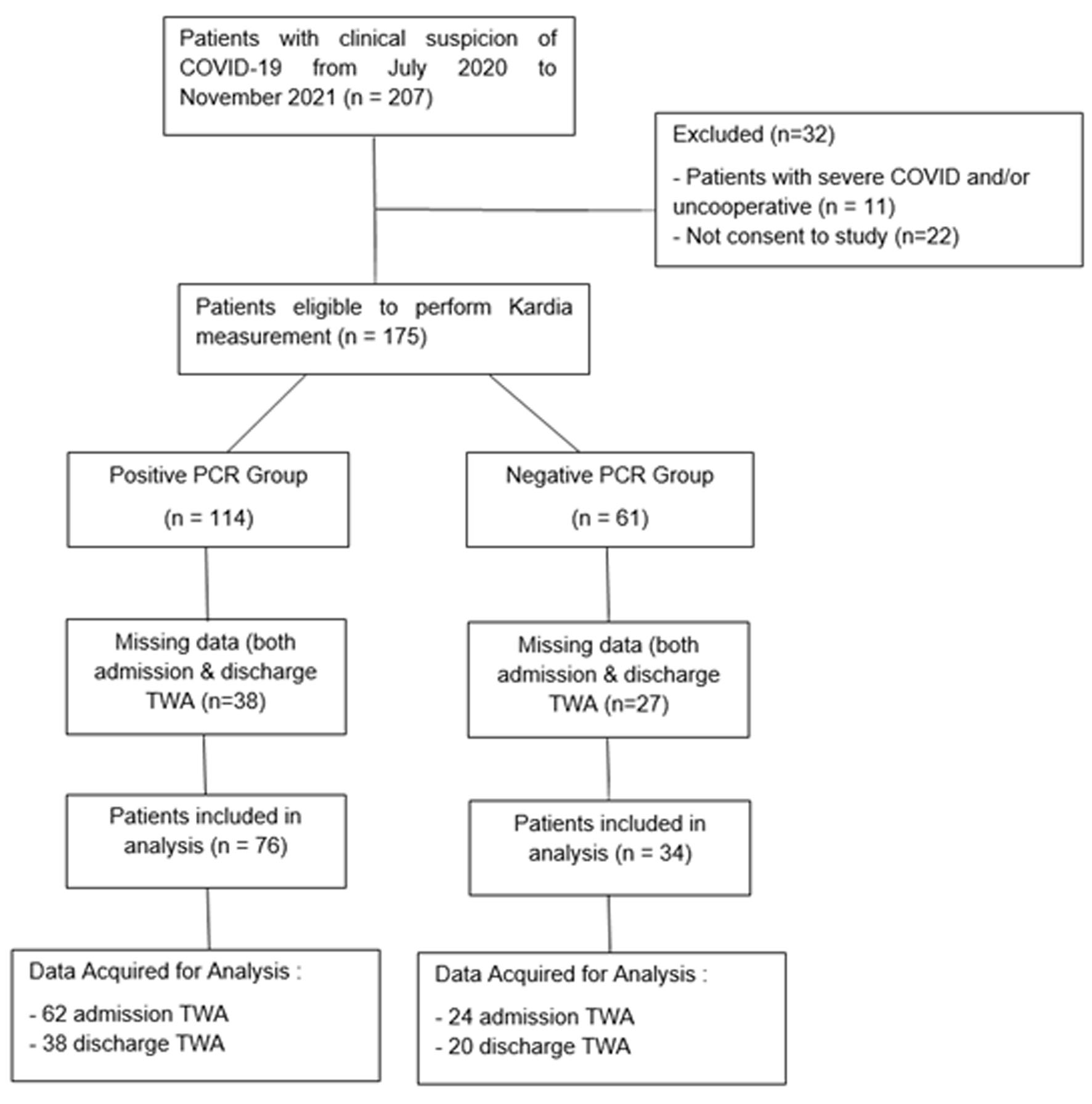 Click for large image | Figure 1. STROBE diagram of the study. |
The baseline characteristics of the study population are summarized in Table 1. Patients included in the study were categorized based on PCR results into PCR-positive group and PCR-negative group; in turn, the PCR-positive group was sub-categorized into mild and moderate severity subgroups. In general, there was no significant difference in baseline characteristics between both groups in respect of age, gender, blood pressure, and body mass index (BMI). No differences were found in the mean value of inflammatory marker C-reactive protein (CRP) and electrolytes level in the COVID-19 group from the control group. Comorbidities such as hypertension, coronary artery disease (CAD), chronic kidney disease (CKD), and diabetes mellitus (DM), were evenly distributed between the two groups. Patients in both groups were treated equally with standard medications such as levofloxacin, azithromycin, and favipiravir. Standard favipiravir doses were also administered for 5 days to some of the patients in the PCR-negative group, based on clinical suspicion of COVID-19 while waiting for the PCR result. However, none of the patients took hydroxychloroquine. The average length of hospital stay was significantly longer in the PCR-positive group.
 Click to view | Table 1. Baseline Characteristics of Study Population |
TWA profile in patients with COVID-19
TWA profiles characterizing COVID-19 patients at hospital admission and discharge are summarized in Table 2. The results indicate that TWA values were similar between PCR-positive and PCR-negative group during admission (42.47 ± 26.52 µV versus 44.72 ± 38.21 µV, P = 0.757). On the contrary, TWA values of PCR-positive group were higher at discharge compared to PCR-negative group (53.45 ± 34.42 µV versus 25.5 ± 17.64 µV, P = 0.030) (Table 2).
 Click to view | Table 2. TWA Profile on Admission and Discharge According to PCR Swab Result* |
TWA and COVID-19 severity
The relation between TWA and COVID-19 illness severity is summarized in Table 3. In the PCR-positive group, 76 patients were analyzed, yielding 62 admission TWA and 38 discharge TWA recordings. On admission, there were 75.80% of patients categorized into mild severity subgroup and 24.19% of patients into moderate severity subgroup. Despite the tendency of higher TWA during discharge in the moderate severity subgroup, there was no statistically significant variability in TWA values during admission (44.29 ± 27.14 µV versus 36.75 ± 24.46 µV, P = 0.341) or discharge (49.47 ± 33.62 µV versus 61.09 ± 35.99 µV, P = 0.330) between mild and moderate COVID-19 severity subgroups.
 Click to view | Table 3. COVID-19 Patients Disease Severity and TWA |
Correlation of positive PCR and TWA at discharge
A linear regression analysis (Fig. 2) shows that PCR result is a variable that has a significant correlation with TWA (P < 0.05), compared to other clinical variables such as medications, comorbidities and electrolytes. After adjustment of confounding variables, a final multivariate model showed that in patients with confirmed COVID-19 at admission, an increment of 18.298 (1.02 - 34.93) points in microvolt TWA at discharge was observed, with a P-value of 0.030 and R square of 0.0810 (Table 4).
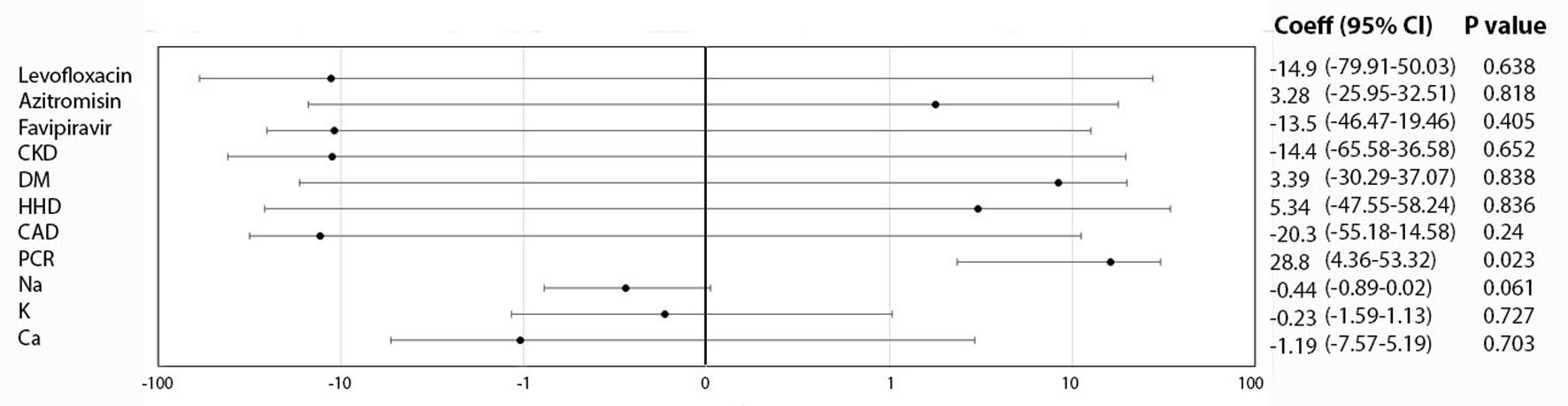 Click for large image | Figure 2. Initial regression model of TWA at discharge and clinical variables. TWA: T-wave alternans. |
 Click to view | Table 4. Final Regression Model of TWA at Discharge and PCR Result |
| Discussion | ▴Top |
This is the first study that addresses the correlation between COVID-19 and TWA as a repolarization heterogeneity marker. Prior data have shown that patients infected with SARS-CoV-2 are more vulnerable to atrial and ventricular arrhythmias. Arrhythmias were present in approximately 44% of patients and associated with poor outcomes and higher mortality [7]. Hypoxia caused by direct viral effects on the lungs and cardiac tissues (myocarditis), myocardial ischemia, abnormal host immune response, and electrolyte imbalances are proposed as potential arrhythmogenesis mechanisms in COVID-19 [10].
The use of proposed COVID-19 medications such as hydroxychloroquine, levofloxacin, azithromycin, etc., results in a concern to monitor QTc prolongation to avoid torsades de pointes (TdP) or other fatal ventricular arrhythmias [25, 26]. However, prolongation of QTc interval also occurs in COVID-19 patients without medication, and a finding by Rubin et al suggested that COVID-19 infection was an independent predictor and significantly associated with the likelihood of prolonged QTc interval (> 500 ms) compared to COVID-19 negative counterparts [27, 28].
Previously, viral infection and inflammatory diseases have been reported to affect repolarization ECG markers [29-31]. Emerging evidence supports the possibility of Ca2+ and K+ channels dysregulation in cardiomyocytes by SARS-CoV-2 genes, resulting in decreased cardiac contractility and heightened susceptibility to arrhythmias, and thus termed viral channelopathies. The interplay between ion channels and the virus is modulated further by excessive inflammation (interleukin-6, interleukin-1, and tumor necrosis factor-α) leading to inflammatory cardiac channelopathies [32]. However, the implementation of manual measurement of repolarization ECG markers in the clinical setting is prone to variability by intra- or inter-observer bias. In addition, differences in determining QTc interval measurement exist in various published studies, such as the heart-rate correction methods by Bezett or Framingham [33].
On the other hand, there is still no universal consensus to measure T-peak to T-end (TpTe), despite the widely accepted tangential method [34]. Furthermore, the global pandemic further complicated cardiology services as COVID-19 eventually emerged as an airborne disease, and physical distancing had been carried out to prevent the spread of infection. Therefore, attempts were made to use portable ECG devices to minimize exposure and disease transmission [18, 35].
TWA has been recognized as an established marker of repolarization heterogeneity at a cellular level and recommended in guidelines as an additional prognostic marker of TdP or ventricular arrhythmias for various cardiovascular disease settings and non-cardiovascular diseases alike [16, 36]. Some studies also highlighted the existence of TWA in channelopathies such as long QT syndrome [37] and early repolarization syndrome [38]. Up to date, there is still no study that specifically assesses the utility of TWA in the context of COVID-19, although several case reports indicate that TWA may exist in infectious disorders, such as Chagas disease and inflammatory illness like systemic lupus erythematosus (SLE) [39, 40].
Our study introduced a method to overcome both measurement subjectivity and disease transmission issue, hence being the first study to evaluate TWA in COVID-19 patients automatically using the novel EAMF method to minimize individual bias while keeping recording time short, by wireless ECG self-acquisition in cooperative patients. Previously, TWA measurement in the clinical setting is traditionally performed by algorithms such as the spectral method and MMA method, mostly integrated into continuous ambulatory ECG (Holter) monitor or stress exercise testing, which are not always suitable in certain situations, such as the COVID-19 pandemic [17].
By using the EAMF method in our study, the recursive extraction of ECG windows allowed the rejection of only some extracted windows in case of the presence of noisy parts without discarding the entire ECG recording and without compromising the reliability of results [21].
The core preprocessing phase of the method is represented by the enhancement phase that implies cancellation of any possible reciprocal influence among other alternans types (such as P-wave alternans (PWA) or QRS-complex alternans), which could bias TWA measurement. Additionally, EAMF method can measure TWA in many different conditions because it adjusts the alternans frequency according to the local heart rate. Eventually, the method filters out the ECG signal components around alternans frequency, resulting in robustness against interferences and noises in most frequency bands, useful in case of ECG acquired by portable or wearable devices and shorter acquisition period as short as 30 s of recording [21].
Our study revealed the dynamics of TWA in patients with suspected COVID-19, indicating increased repolarization heterogeneity during disease progression in the PCR-positive group. Even though the baseline TWA levels were similar between both groups during admission (42.47 ± 26.52 µV vs. 44.72 ± 38.21 µV), higher TWA levels were observed during discharge in the PCR-positive compared to the PCR-negative group (53.45 ± 34.42 µV vs. 25.15 ± 17.64 µV, P = 0.03). Furthermore, there was a significant correlation between positive PCR result and TWA value, after adjustment of other confounding variables (R2 = 0.081, P = 0.030). This finding is in agreement with earlier meta-analyses that have described the effect of COVID-19 on various ECG indicators of repolarization heterogeneity [5, 15].
ECG markers that reflect repolarization abnormalities such as TpTe interval, TpTe/QTc ratio, and index of cardiac electrophysiology balance (iCEB) tend to increase significantly in COVID-19 patients [5]. The escalation of iCEB indicated that patients with COVID-19 are predisposed to TdP-mediated ventricular tachycardia or ventricular fibrillation, emphasizing the role of repolarization disturbance in its arrhythmogenesis [41, 42]. A meta-analysis has demonstrated that initial ECG on admission, including QTc prolongation is associated with short-term, in-hospital poor outcome [43]. Our study suggests that serial evaluation of ECG markers is also important, rather than taking a single ECG recording during admission. Repolarization abnormalities, contributed by the viral infection may happen during the natural course of COVID-19 progression, only to be noticeable at patient discharge from the hospital. Another study by Vandenberk et al corresponds to this finding that repolarization marker abnormalities at discharge may predict mortality and re-admission for up to 1-year follow-up [14]. Although TWA increment at discharge was evident in COVID-19 patients, our study did not find any significant difference in TWA levels between mild and moderate severity subgroups in patients with COVID-19, both during admission (46.95 ± 31.35 µV vs. 38.25 ± 22.1 µV, P = 0.45) and discharge (46.89 ± 32.98 µV vs. 69.90 ± 33.91 µV, P = 0.14). Another study by Koc et al demonstrated that other ventricular repolarization parameters such as TpTe interval, TpTe/QT ratio, and TpTe/QTc ratio were more prominently increased in the COVID-19 subgroup complicated by severe pneumonia, which is a scenario involving critical illness and other causes of inflammation and comorbidities [44]. Our study discovered evidence that repolarization abnormalities may ensue even in mild to moderate COVID-19 clinical severity, adding to the possibility of a direct viral channelopathy effect.
Study limitations
Several limitations of this study should be considered. Firstly, this is a single-center study with a relatively small sample size. Albeit not statistically significant, some heterogeneities such as the imbalance usage of medications that may prolong QT interval in the COVID-19 group, can become a potential confounder for this study. Secondly, our study did not include patients with severe COVID-19 subgroup. Further studies of TWA in a wider spectrum of COVID-19 patients by analyzing other ECG acquisition data system may be able to collect signals more passively, compared to patient-activated commercial portable ECG devices [45]. Follow-up of patients with elevated TWA and the correlation with subsequent arrhythmias and other adverse events can also become point of interests in future studies for the involvement of cardiac repolarization abnormalities in long COVID-19 syndrome.
Conclusions
Our study shows that TWA value is significantly increased on follow-up ECG obtained during patient discharge in the PCR-positive group. Repolarization heterogeneity as represented by TWA can occur even in mild and moderate severity of COVID-19, albeit without significant relationship between severity and TWA in these subgroups.
Acknowledgments
The authors wish to dedicate this manuscript to all healthcare staff in Mohammad Hoesin General Hospital who are involved in the fight against COVID-19.
Financial Disclosure
This study is partially supported by the Indonesian Heart Rhythm Society (InaHRS) for publication fee only.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Alexander Edo Tondas: conceptualization, data curation, formal analysis, methodology, writing - original draft. Dian Andina Munawar: conceptualization, formal analysis, writing - review and editing. Ilaria Marcantoni: conceptualization, method analysis, writing - review and editing. Iche Andriyani Liberty: data curation, formal analysis, writing - original draft. Rido Mulawarman: data curation, formal analysis, writing - original draft. Muhammad Hadi: data curation, writing - original draft. Monica Trifitriana: data curation, writing - original draft. Taufik Indrajaya, Muhammad Yamin, and Irfannuddin: writing - review and editing. Laura Burrattini: conceptualization, method analysis, writing - review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
TWA: T-wave alternans; TpTe: T-peak to T-end; QTc: QT corrected; iCEB: index of cardiac electrophysiology balance; ECG: electrocardiogram; EAMF: enhanced adaptive match filter; PCR: polymerase chain reaction; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure
| References | ▴Top |
- Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83(3):217-220.
doi pubmed - BNPB. Update Infografis percepatan penanganan COVID-19 di Indonesia per tanggal 18 Januari 2022 Pukul 12.00 WIB. BNPB. 2022.
- Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543-558.
doi pubmed - Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med. 2021;44:352-357.
doi pubmed - Tondas AE, Mulawarman R, Trifitriana M, Nurmaini S, Irfannuddin I. Arrhythmia risk profile and ventricular repolarization indices in COVID-19 patients: a systematic review and meta-analysis. J Infect Dev Ctries. 2021;15(2):224-229.
doi pubmed - Nurmaini S, Tondas AE, Partan RU, Naufal M, Darmawahyuni A, Firdaus F, et al. Automated detection of COVID-19 infected lesion on computed tomography images using faster-RCNNs. Eng Lett. 2020;28(4):EL_28_4_38.
- Pranata R, Huang I, Raharjo SB. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2020;20(5):193-198.
doi pubmed - Yuniadi Y, Yugo D, Fajri M, Tejo BA, Widowati DR, Hanafy DA, Raharjo SB. ECG characteristics of COVID-19 patient with arrhythmias: Referral hospitals data from Indonesia. J Arrhythm. 2022;38(3):432-438.
doi pubmed - Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583-590.
doi pubmed - Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, Tedrow U. Arrhythmias and COVID-19: a review. JACC Clin Electrophysiol. 2020;6(9):1193-1204.
doi pubmed - Bakhshaliyev N, Ozdemir R. The impact of hydroxychloroquine-azithromycin combination on Tpeak-to-end and Tpeak-to-end/QT ratio during a short treatment course. Ann Noninvasive Electrocardiol. 2021;26(4):e12846.
doi pubmed - Yenercag M, Arslan U, Dogdus M, Gunal O, Ozturk CE, Aksan G, Erdogan G, et al. Evaluation of electrocardiographic ventricular repolarization variables in patients with newly diagnosed COVID-19. J Electrocardiol. 2020;62:5-9.
doi pubmed - Vandenberk B, Van De Sijpe G, Ingelaere S, Engelene M, Vermeulen J, Verhamme P, et al. Repolarization abnormalities at admission predict 30-day outcome in COVID-19. EP Eur. 2021;23(Suppl 3):euab116.013.
doi - Vandenberk B, Engelen MM, Van De Sijpe G, Vermeulen J, Janssens S, Vanassche T, Verhamme P, et al. Repolarization abnormalities on admission predict 1-year outcome in COVID-19 patients. Int J Cardiol Heart Vasc. 2021;37:100912.
doi pubmed - Mahmoudi E, Mollazadeh R, Mansouri P, Keykhaei M, Mirshafiee S, Hedayat B, Salarifar M, et al. Ventricular repolarization heterogeneity in patients with COVID-19: Original data, systematic review, and meta-analysis. Clin Cardiol. 2022;45(1):110-118.
doi pubmed - Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, Ikeda T, et al. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility—consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol. 2011;58(13):1309-1324.
doi pubmed - Aro AL, Kentta TV, Huikuri HV. Microvolt T-wave alternans: where are we now? Arrhythm Electrophysiol Rev. 2016;5(1):37-40.
doi pubmed - Tondas AE, Halim RA, Guyanto M. Minimal or no touch electrocardiography recording and remote heart rhythm monitoring during covid-19 pandemic era. Indones J Cardiol. 2020;41(2):133-141.
doi - Pranata R, Tondas AE, Huang I, Lim MA, Siswanto BB, Meyer M, Mitrovic V. Potential role of telemedicine in solving ST-segment elevation dilemmas in remote areas during the COVID-19 pandemic. Am J Emerg Med. 2021;42:242-243.
doi pubmed - Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar P, Boriani G, et al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace. 2022;24(6):979-1005.
doi pubmed - Marcantoni I, Sbrollini A, Morettini M, Swenne CA, Burattini L. Enhanced adaptive matched filter for automated identification and measurement of electrocardiographic alternans. Biomed Signal Process Control [Internet]. 2021;68:102619.
doi - Ghaferi AA, Schwartz TA, Pawlik TM. STROBE reporting guidelines for observational studies. JAMA Surg. 2021;156(6):577-578.
doi pubmed - Steel PAD, Carpenter CR, Fengler B, Cantrill S, Schneider S. Calculated decisions: ACEP ED COVID-19 management tool. Emerg Med Pract. 2021;23(Suppl 7):CD1-CD6.
- National Institutes of Health. Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19). Nih. 2021;2019:1-243.
- Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc-prolonging and Torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin Proc. 2020;95(6):1213-1221.
doi pubmed - Purwowiyoto SL, Hermanto DY, Iqbal M. Managing QT prolongation in the era of coronavirus disease 2019 (COVID-19). Indones J Cardiol. 2020;41(2):108-111.
doi - Changal K, Paternite D, Mack S, Veria S, Bashir R, Patel M, Soni R, et al. Coronavirus disease 2019 (COVID-19) and QTc prolongation. BMC Cardiovasc Disord. 2021;21(1):158.
doi pubmed - Rubin GA, Desai AD, Chai Z, Wang A, Chen Q, Wang AS, Kemal C, et al. Cardiac corrected QT interval changes among patients treated for COVID-19 infection during the early phase of the pandemic. JAMA Netw Open. 2021;4(4):e216842.
doi pubmed - Gunes HM, Babur Guler G, Guler E, Demir GG, Teber MK, Kizilirmak F, Cakal B, et al. Assessment of repolarization abnormalities in baseline electrocardiograms of patients with myocarditis. Turk J Med Sci. 2017;47(5):1333-1339.
doi pubmed - Wu KC, Bhondoekhan F, Haberlen SA, Ashikaga H, Brown TT, Budoff MJ, D'Souza G, et al. Associations between QT interval subcomponents, HIV serostatus, and inflammation. Ann Noninvasive Electrocardiol. 2020;25(2):e12705.
doi - Goldeli O, Dursun E, Komsuoglu B. Dispersion of ventricular repolarization: a new marker of ventricular arrhythmias in patients with rheumatoid arthritis. J Rheumatol. 1998;25(3):447-450.
- Etheridge SP, Asaki SY. COVID-19 infection and corrected QT interval prolongation - collateral damage from our newest enemy. JAMA Netw Open. 2021;4(4):2021-2023.
doi pubmed - Abellas-Sequeiros M, Lozano-Granero C, Garcia-Sebastian C, Franco-Diez E, Hernandez-Madrid A, Moreno-Planas J, Masjuan-Vallejo J, et al. Monitoring of QTc interval in patients with COVID-19. First experience with a portable ECG-recording device. Cardiol J. 2021;28(3):483-485.
doi pubmed - Rosenthal TM, Masvidal D, Abi Samra FM, Bernard ML, Khatib S, Polin GM, Rogers PA, et al. Optimal method of measuring the T-peak to T-end interval for risk stratification in primary prevention. Europace. 2018;20(4):698-705.
doi pubmed - Minguito-Carazo C, Echarte-Morales J, Benito-Gonzalez T, Del Castillo-Garcia S, Rodriguez-Santamarta M, Sanchez-Munoz E, Maniega CG, et al. QT interval monitoring with handheld heart rhythm ECG device in COVID-19 patients. Glob Heart. 2021;16(1):42.
doi pubmed - Verrier RL. Sex-based differences in T-wave alternans. Sex and cardiac electrophysiology. Elsevier Inc. 2020:141-152.
doi - Takasugi N, Goto H, Takasugi M, Verrier RL, Kuwahara T, Kubota T, Toyoshi H, et al. Prevalence of Microvolt T-Wave Alternans in Patients With Long QT Syndrome and Its Association With Torsade de Pointes. Circ Arrhythm Electrophysiol. 2016;9(2):e003206.
doi pubmed - Tondas AE, Batubara EAD, Sari NY, Marcantoni I, Burattini L. Microvolt T-wave alternans in early repolarization syndrome associated with ventricular arrhythmias: A case report. Ann Noninvasive Electrocardiol. 2022;28(1):e13005.
doi pubmed - Harada M, Motoki H, Kashima Y, Nakamura C, Hashizume N, Kishida D, Imamura H, et al. T-wave alternans in a case with systemic lupus erythematosus-related myocarditis. J Cardiol Cases. 2018;18(4):119-122.
doi pubmed - Raadschilders L, Barbosa MP, Carmo AA, Nouwen JL, Rocha MO, Ribeiro AL. Microvolt T-wave alternans in Chagas disease. Int J Cardiol. 2015;187:7-8.
doi pubmed - Robyns T, Lu HR, Gallacher DJ, Garweg C, Ector J, Willems R, Janssens S, et al. Evaluation of Index of Cardio-Electrophysiological Balance (iCEB) as a New Biomarker for the Identification of Patients at Increased Arrhythmic Risk. Ann Noninvasive Electrocardiol. 2016;21(3):294-304.
doi pubmed - Mulawarman R, Ramadhan MS, Trifitriana M, Mulawarman H, Nurmansyah MI, Halim RA, et al. Index of Cardiac Electrophysiological Balance (iCEB) in patients with COVID-19. Eur Hear J Suppl. 2021;23(Issue Supplement_F):7-8.
doi - Alsagaff MY, Oktaviono YH, Dharmadjati BB, Lefi A, Al-Farabi MJ, Gandi P, Marsudi BA, et al. Electrocardiography on admission is associated with poor outcomes in coronavirus disease 2019 (COVID-19) patients: A systematic review and meta-analysis. J Arrhythm. 2021;37(4):877-885.
doi pubmed - Koc M, Sumbul HE, Gulumsek E, Koca H, Bulut Y, Karakoc E, Turunc T, et al. Disease severity affects ventricular repolarization parameters in patients with COVID-19. Arq Bras Cardiol. 2020;115(5):907-913.
doi pubmed - Koo CH, Lee HC, Kim TK, Cho YJ, Nam K, Choi EK, Choi SN, et al. Microvolt T-wave alternans at the end of surgery is associated with postoperative mortality in cardiac surgery patients. Sci Rep. 2019;9(1):17351.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


