| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 3, June 2022, pages 162-171
Outcomes of Heart Failure in COVID-19 Patients: An Appalachian Experience
Huzefa Bhopalwalaa, i, Aelia Akbara, Nakeya Dewaswalab, Lauren Wisnieskic, Abdul Mannan Khan Minhasd, Akbar Hussaine, Vinayak Mishraf, Sourbha S. Danig, Andrew Kolodziejb, Gaurang Vaidyab, Abhishek Kulkarnih, Jonathan Piercya, Shyam Gantia, Nagabhishek Mokaa, Adnan Bhopalwalaa
aDepartment of Internal Medicine, Appalachian Regional Healthcare, Whitesburg, KY, USA
bDepartment of Cardiovascular Disease, University of Kentucky, Lexington, KY, USA
cDepartment of Public Health and Research, Lincoln Memorial University, Harrogate, TN, USA
dDepartment of Internal Medicine, Forrest General Hospital, Hattiesburg, MS, USA
eJinnah Sindh Medical University, Karachi, Pakistan
fGrant Government Medical College and Sir J.J. Group of Hospitals, Mumbai, India
gDepartment of Cardiovascular Disease, Lahey Hospital and Medical Center, Burlington, MA, USA
hDivision of Cardiology, Department of Internal Medicine, Southern Illinois University School of Medicine, Springfield, IL, USA
iCorresponding Author: Huzefa Bhopalwala, Department of Internal Medicine, Appalachian Regional Healthcare, Whitesburg, KY 41858, USA
Manuscript submitted April 21, 2022, accepted May 14, 2022, published online June 2, 2022
Short title: HF Outcomes in COVID-19
doi: https://doi.org/10.14740/cr1389
| Abstract | ▴Top |
Background: The Southeastern rural areas of the USA have a higher prevalence of heart failure (HF). Coronavirus disease 2019 (COVID-19) infection is associated with poor outcomes in patients with HF. Our study aimed to compare the outcomes of hospitalized HF patients with and without COVID-19 infection specifically in rural parts of the USA.
Methods: We conducted a retrospective cohort study of HF patients with and without COVID-19 hospitalized in Southeastern rural parts of the USA by using the Appalachian Regional Healthcare System. Analyses were stratified by waves from April 1, 2020 to May 31, 2021, and from June 1, 2021 to October 19, 2021.
Results: Of the 14,379 patients hospitalized with HF, 6% had concomitant COVID-19 infection. We found that HF patients with COVID-19 had higher mortality rate compared to those without COVID-19 (21.8% versus 3.8%, respectively, P < 0.01). Additionally, hospital resource utilization was significantly higher in HF patients with COVID-19 compared to HF patients without COVID-19 with intensive care unit (ICU) utilization of 21.6% versus 13.8%, P < 0.01, mechanical ventilation use of 17.3% versus 6.2%, P < 0.01, and vasopressor/inotrope use of 16.8% versus 7.9%, P < 0.01. A lower percentage of those with COVID-19 were discharged home compared to those without a COVID-19 diagnosis (63.4% versus 72.0%, respectively). There was a six-fold greater odds of dying in the first wave and seven-fold greater odds of dying in the second wave.
Conclusions: Our study confirms previous findings of poor outcome in HF patients with COVID-19. There is a need for review of healthcare resources in rural hospitals which already face numerous healthcare challenges.
Keywords: Coronavirus; Heart failure; COVID-19; SARS-CoV-2; Cohort; Mortality; Survival
| Introduction | ▴Top |
Since December of 2019, coronavirus disease 2019 (COVID-19) has been a major cause of morbidity and mortality around the world. As of October 19, 2021, 242 million cases and 4.9 million deaths have been reported worldwide from COVID-19 [1]. Patients with comorbidities, especially cardiovascular diseases, are more susceptible to developing a severe form of disease [2]. Several studies have demonstrated that the cardiovascular risk factors such as advanced age, hypertension, diabetes, and obesity are also significant risk factors for severe COVID-19 infection [3]. Conversely, patients with COVID-19 are at increased risk of a broad range of cardiovascular disorders including cerebrovascular disorders, dysrhythmias, ischemic and nonischemic heart disease, pericarditis, myocarditis, heart failure (HF), and thromboembolic disease [4].
In an analysis of the Premier Healthcare database, which is a multi-center all-payer US database, Bhatt et al found that patients with preexisting HF hospitalized with COVID-19 had a higher mortality (24.2%) rates as compared to those hospitalized with HF without COVID-19 (2.6%) and those hospitalized for other reasons (4.6%) [5]. Further, a comparison study by Chatrath et al in London, UK found that patients hospitalized for HF with COVID-19 infection had a significantly higher rate of inpatient mortality as compared to those without COVID-19 infection (50.0% vs. 10.6%; P < 0.001) [6].
The rural parts of the USA have higher rates of mortality due to HF as compared to other parts of the USA [7, 8]. We aimed to compare the outcomes of HF patients hospitalized with concomitant COVID-19 infection as compared to those without COVID-19 in the rural healthcare system of USA.
| Materials and Methods | ▴Top |
This is a multi-center retrospective cohort study of hospitalized patients with a primary or secondary diagnosis of HF between April 1, 2020 and October 19, 2021. The study was approved by the Appalachian Regional Healthcare Institutional Review Board (IRB). As per IRB requirements, written consent was waived for this project as it is a retrospective study that includes the abstraction of data from medical records. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
The data were extracted from 13 facilities in the Appalachian Regional Healthcare System in Southeastern parts of Kentucky and in West Virginia that share the same electronic health record. The principal admitting diagnosis of HF using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes in patients 18 years or older was identified. The primary exposure of interest was COVID-19 diagnosis. The primary outcome was in-hospital mortality and secondary outcomes included intensive care unit (ICU) stay, mechanical ventilation, length of stay (LOS), re-admittance between 1 and 30 days, and re-admittance between 31 and 60 days. Records were excluded from patients that were < 18 years old or were missing information on COVID-19 diagnosis, age, or discharge status. A readmission was defined as a subsequent hospital admission for any cause a specified time interval following an initial index admission.
Descriptive statistics were utilized to compare demographic characteristics, comorbidities, discharge status, and resource utilization between those hospitalized with HF that had a COVID-19 diagnosis and those that did not have a COVID-19 diagnosis using Chi-square analyses for categorical variables. Fisher exact was used instead of Chi-square analyses when expected cell counts were < 5. Independent t-tests were used to compare the average age and body mass index (BMI) between HF patients with and without a COVID-19 diagnosis.
Outcomes among HF patients with and without a COVID-19 diagnosis were compared using adjusted and unadjusted regression models. Mixed-effects logistic regression was used for all binary outcomes (mortality, ICU stay, mechanical ventilation, re-admittance between 1 and 30 days, and re-admittance between 31 and 60 days) and mixed linear regression was used for LOS. A random intercept for hospital (n = 13) was included in all models to adjust for shared variance at the hospital level. Adjusted analyses were adjusted for age, gender, marital status, obesity, diabetes mellitus, hypertension, pulmonary disease, kidney disease, and smoking. Analyses were stratified by waves, which were identified as wave 1 (April 1, 2020 to May 31, 2021) and wave 2 (June 1, 2021 to October 19, 2021) using histograms of the case count over time.
Additional models were built among HF patients to identify demographic and comorbidity risk factors associated with the primary and secondary outcomes among those with a COVID-19 diagnosis. Risk factors included age, gender, marital status, obesity, diabetes mellitus, hypertension, pulmonary disease, kidney disease, smoking, and wave. For mixed linear regression models and t-tests, normality and homoscedasticity of residuals were checked using residual plots. LOS and BMI did not follow a normal distribution; therefore, they were transformed by the logarithm function. Model results were back-transformed to their original scale for ease of interpretation.
| Results | ▴Top |
In total, 14,408 records were downloaded from the database. After exclusion criteria (< 18 years old or missing age (n = 14), missing COVID-19 diagnosis information (n = 0), missing discharge data (n = 15)), 14,379 records remained. Out of the total hospitalized with HF, 6.0% were diagnosed with COVID-19 (Fig. 1). Patients with a COVID-19 diagnosis had a higher mortality rate compared to those without a COVID-19 diagnosis (21.8% versus 3.8%, respectively, P < 0.01). Of those that were discharged, a lower percentage of those with a COVID-19 diagnosis were discharged home compared to those without a COVID-19 diagnosis (63.4% versus 72.0%, respectively) and remaining were referred to skilled nursing facilities, rehabilitation facilities, hospice, and other locations.
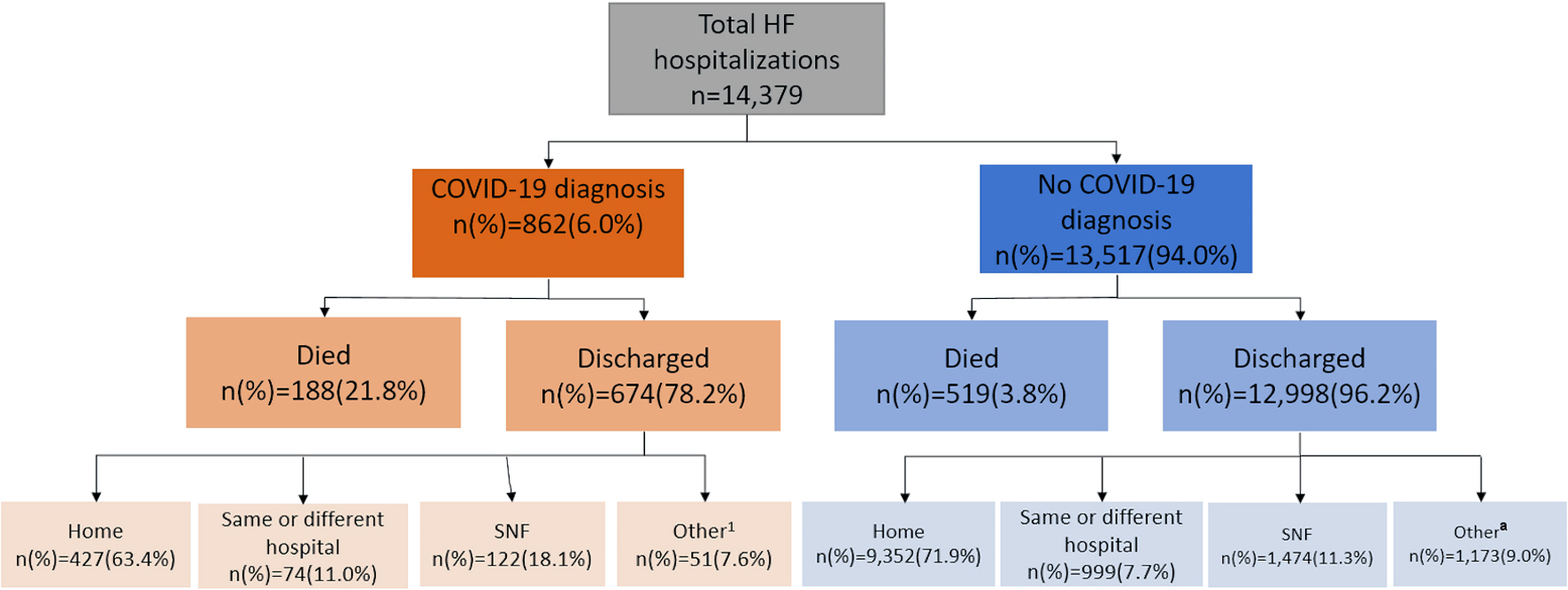 Click for large image | Figure 1. Summary of patients hospitalized with HF during the COVID-19 pandemic. aIncludes intermediate care facilities, court/law, hospice, rehabilitation facilities, and other stated locations. HF: heart failure; COVID-19: coronavirus disease 2019; SNF: skilled nursing facility. |
Table 1 shows the baseline characteristics of HF patients with and without COVID-19 infection. HF patients hospitalized with a COVID-19 diagnosis were older (70.5 years versus 69.2 years, P < 0.01), more likely to be male (50.2% versus 45.5%, P < 0.01), had a higher mean BMI (32.6 kg/m2 versus 31.8 kg/m2, P = 0.03), less likely to have a pulmonary disease (52.6% versus 58.0%, P < 0.01), malignancy (3.1% versus 4.7%, P = 0.04), and were less likely to smoke (30.5% versus 41.1%, P < 0.01) compared to the patients without COVID-19 diagnosis.
 Click to view | Table 1. Baseline Characteristics of a Sample of Hospitalized HF Patients Stratified by COVID-19 Diagnosis Status (N = 14,379) |
Overall, resource use as defined as the percent utilization over total HF hospitalizations was lower in HF hospitalizations with COVID-19 compared to HF hospitalizations without COVID-19 (Fig. 2). However, the percent of HF hospitalization with COVID-19 that required an arterial catheter (1.3% versus 0.6%, P = 0.01), ICU utilization (21.6% versus 13.8%, P < 0.01), mechanical ventilation (17.3% versus 6.2%, P < 0.01), and vasopressor/inotrope use (16.8% versus 7.9%, P < 0.01) was significantly higher compared to HF hospitalizations without a COVID-19 diagnosis.
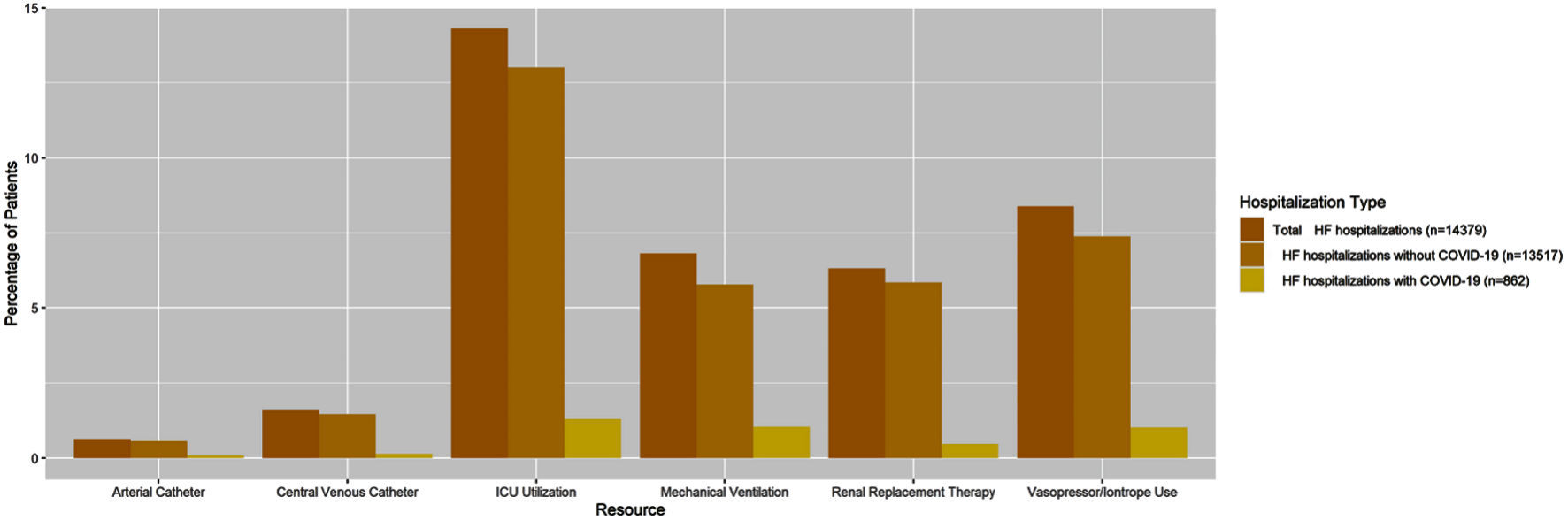 Click for large image | Figure 2. Hospital resource use of as a percentage of total HF patients by COVID-19 diagnosis status (n = 14,379). HF: heart failure; COVID-19: coronavirus disease 2019; ICU: intensive care unit. |
In Table 2, unadjusted and adjusted analyses indicate that HF patients with a COVID-19 diagnosis had higher odds of inpatient mortality, mechanical ventilation use, and requiring ICU care compared to those without a COVID-19 diagnosis. In addition, patients with a COVID-19 diagnosis had greater LOS compared with patients without a COVID-19 diagnosis. Results were inconstant for re-admittance between 1 and 30 days as HF patients with COVID-19 had significantly higher odds of being re-admitted between 1 and 30 days with odds ratio (OR): 1.25, confidence interval (CI): 1.01 - 1.54, P value < 0.05 during the first wave in adjusted analyses, compared with OR: 1.18, CI: 0.86 - 1.60 in second wave. HF patients with COVID-19 had lower odds of being re-admitted between 31 and 60 days compared with HF patients without COVID-19 during both waves. Results were consistent across both the waves for most of the outcomes as shown in the Table 2.
 Click to view | Table 2. Adjusted and Unadjusted Results for the Association of COVID-19 Diagnosis and Multiple Outcomes in a Sample of Hospitalized HF Patients (N = 14,379) |
Among HF patients with a COVID-19 diagnosis, there were no significant demographic or comorbidity risk factors identified for needing mechanical ventilation or ICU care (Fig. 3). However, patients that were 75 or older had approximately twice the odds of mortality compared to those who were < 65 years old (OR (95% CI): 2.05 (1.28 - 3.28)).
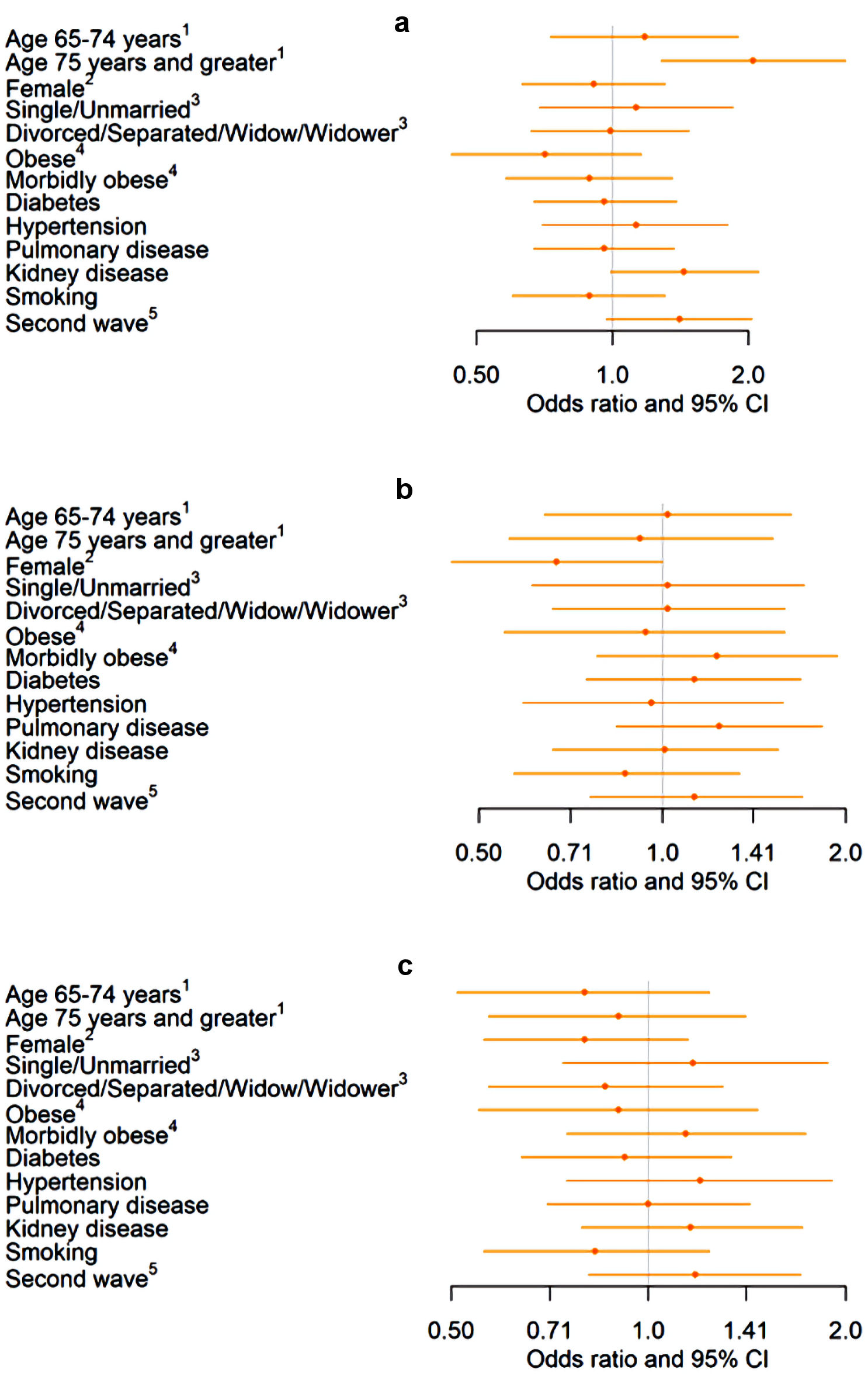 Click for large image | Figure 3. Association of demographics and comorbidities with mortality, mechanical ventilation, and ICU stay in a sample of HF patients diagnosed with COVID-19 (n = 862). (a) Association between demographics and comorbidities and mortality among hospitalized COVID-19 patients. (b) Association between demographics and comorbidities and mechanical ventilation among hospitalized COVID-19 patients. (c) Association between demographics and comorbidities and ICU stay among hospitalized COVID-19 patients. 1Reference: age < 65 years. 2Reference: male. 3Reference: married. 4Reference: not obese. 5Reference: first wave. HF: heart failure; COVID-19: coronavirus disease 2019; ICU: intensive care unit; CI: confidence interval. |
Patients that had kidney disease had about twice the odds of being re-admitted between 1 to 30 days compared to those without kidney disease (1.92 (1.30 - 2.84)) (Fig. 4). Being female and having diabetes were associated with higher odds of being re-admitted between 31 and 60 days (1.93 (1.18 - 3.16)). Patients in the second wave had about 50% lower odds of being re-admitted between 31 and 60 days (0.49 (0.28 - 0.86)). On average, patients aged 75 and older spent approximately 2 more days in the hospital compared with those < 65 years old. Patients with diabetes spent approximately 1 more day in the hospital compared to those without diabetes.
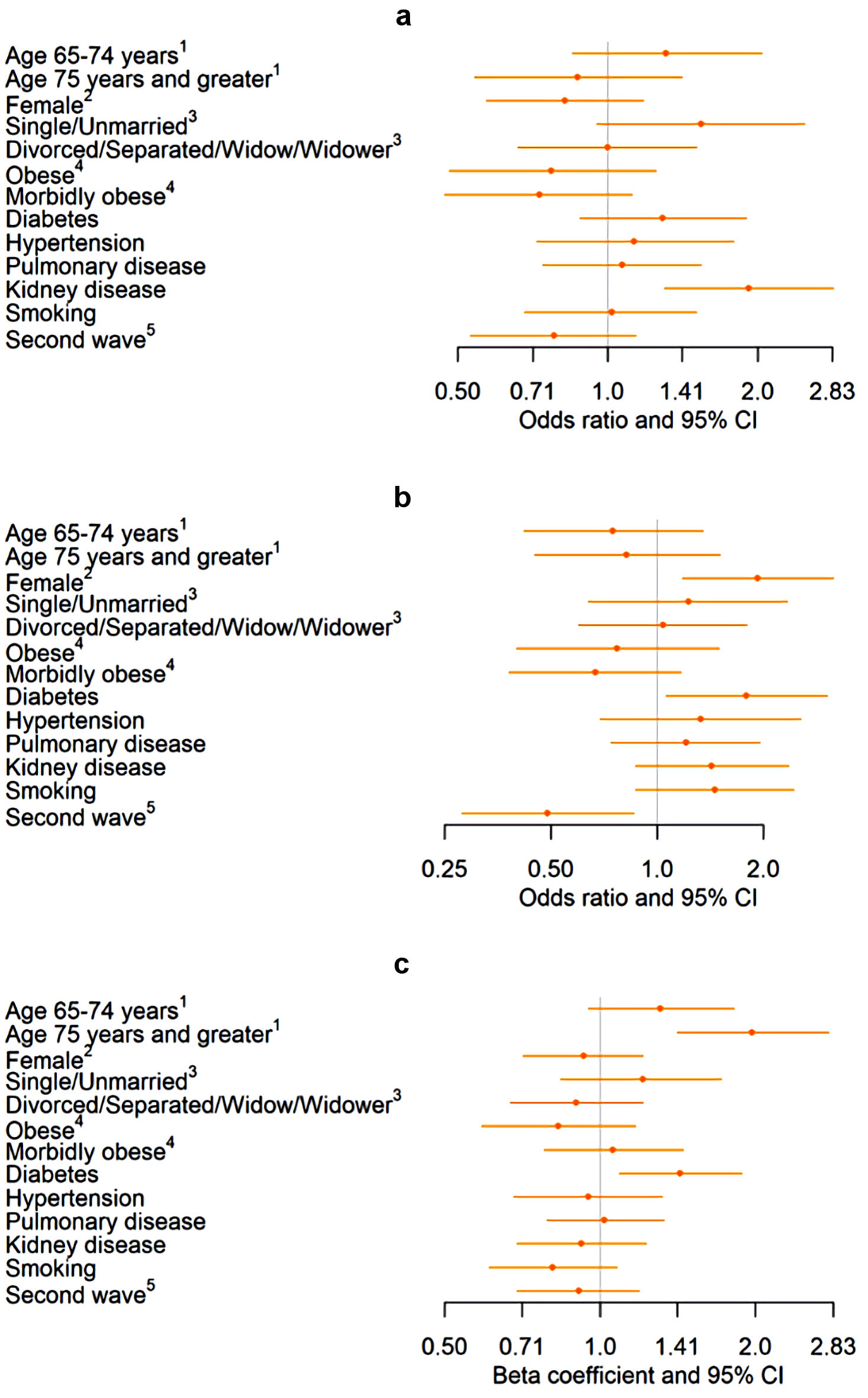 Click for large image | Figure 4. Association of demographics and comorbidities with re-admittance between 1 and 30 days, re-admittance between 31 and 60 days, and length of stay in a sample of HF patients diagnosed with COVID-19 (n = 862). (a) Association between demographics and comorbidities and re-admittance between 1 and 30 days among hospitalized COVID-19 patients. (b) Association between demographics and comorbidities and re-admittance between 31 and 60 days among hospitalized COVID-19 patients. (c) Association between demographics and comorbidities and length of stay among hospitalized COVID-19 patients. Values back-transformed from log10 scale. 1Reference: age < 65 years. 2Reference: male. 3Reference: married. 4Reference: not obese. 5Reference: first wave. HF: heart failure; COVID-19: coronavirus disease 2019; CI: confidence interval. |
| Discussion | ▴Top |
To our knowledge, this is the first study to compare the clinical outcomes in HF patients with and without COVID-19 in rural areas of the USA. In this analysis of 14,379 patients with HF hospitalized during the pandemic, 6% had concomitant COVID-19 infection, and one in five HF patients with COVID-19 died.
Earlier in the pandemic from April to September, 2020, a study from a larger sample of multi-center US healthcare showed similar results, with 6% hospitalized HF patients having concomitant COVID-19 infection and a mortality rate of 24.2% among HF patients with concomitant COVID-19 [5]. The increase in mortality in HF patients with COVID-19 is due to the bidirectional relationship between HF and COVID-19 infection [9-12]. The virus enters the cells through angiotensin-converting enzyme 2 (ACE-2) receptors which are abundant on heart, kidney, and lung alveolar epithelial cells [13]. In turn, dysregulation of the renin-angiotensin-aldosterone system (RAAS) as in patients with HF play a role in the severity of COVID-19 infection [14-16]. It has been hypothesized earlier in the pandemic that patients taking ACE inhibitors or angiotensin receptor blockers would be at a higher risk of adverse clinical outcomes; however, later studies have refuted this hypothesis [17, 18]. In addition, COVID-19 is associated with myocardial injury [19, 20], thrombocytopenia [21, 22], and liver dysfunction [23-27] which predicts higher mortality in the general population and in patients with HF [28, 29].
In our study, HF patients with COVID-19 were likely to be male and recent studies have shown that ACE-2 levels are 50% higher in males with HF [30, 31]. Our study showed that patients with HF and COVID-19 were less likely to be smokers, have pulmonary disease and malignancy as compared to those without COVID-19. In our sample of HF patients, those who were current smokers had a lower incidence of COVID-19 infection as compared to those who were non-smokers (Table 1). Studies relating to COVID-19 and smoking have shown a similar inverse association [32, 33]. There is biological plausibility for this relationship such as nicotine binds to ACE-2 receptors, the binding sites for COVID-19, and also downregulates them in multiple organs [34, 35]. However, previous studies have also shown that smokers who get COVID-19 have overall poor outcomes and increased mortality compared to non-smokers [36].
We found that there was six-fold greater odds of dying in the first wave from April 2020 to May 2021, and seven-fold greater odds of dying in the second wave from June 2021 to October 2021 in HF patients with COVID-19 compared to those hospitalized without concomitant COVID-19 infection (Table 2). The reason for this increase in mortality in HF patients with COVID-19 compared to those without COVID-19 is multifold. First, as shown in a study before the onset of the pandemic there is a higher mortality rate from HF in rural areas as compared to urban areas with occurrence of “heart failure belt” in Southeastern USA [8]. Second, as the COVID-19 mortality has declined nationally, this decline is only seen in urban areas whereas in rural areas the mortality from COVID-19 has doubled [37, 38]. This is due to multiple factors as the vaccination rates for COVID-19 is lower in rural than urban areas [37, 39, 40], and prevalence of obesity is higher in rural than in urban areas [41]. Additionally, healthcare facilities in rural areas are typically less-resourced with reduced access to ventilators and ICU beds which are the key aspects of care for critically ill COVID-19 patients [42, 43]. In addition, rural areas are associated with poor compliance and follow-up that complicate care [29, 44]. Our study shows that there was increased use of resources during and after hospitalization such as mechanical ventilation, ICU stays, skilled nursing and rehabilitation as well as a prolonged hospital stay among HF patients with COVID-19.
Limitations
This study has several limitations, most of which are inherent to the analysis of administrative databases. Since the data are collected based on administrative codes, it is not possible to establish whether a complication was present on admission or developed during the hospital stay. Rare complications of COVID-19 were not reported in this study [45, 46]. Second, laboratory data and metrics like SOFA score, APACHE II, and SAPS III that have been validated to predict mortality in critically ill patients were not evaluated in our study [47]. We were not able to examine the effect of different treatments on the population [44, 48-50]. We were unable to compare outcomes based on medications that the patients were prescribed for HF prior to admission, thereby, we were unable to determine the effects of guideline-directed medical therapy on the severity of COVID-19 infection. Lastly, this study did not distinguish between stages or subcategories of HF. Despite these limitations, this study addresses a significant knowledge gap as a contemporary epidemiological study of COVID-19 in rural regions.
Conclusions
Our study shows that COVID-19 represents a clinical challenge in patients with HF in the setting of rural facilities [51]. This study highlights the need for a review of healthcare challenges in rural areas posed by the concurrence of these two illnesses.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The study was approved by the Appalachian Regional Healthcare institutional review board (IRB). As per IRB requirements, written consent was waived for this project as it is a retrospective study that includes the abstraction of data from medical records.
Author Contributions
HB, AA, ND, AH, VM, LW and AMKM contributed to study design, literature review, statistical analysis, and drafting manuscript. SSD, A. Kolodziej, GV, A. Kulkarni, JP, SG and AB contributed to final approval, mentorship, manuscript revision and intellectual revisions. NM contributed to mentorship and drafting.
Data Availability
The data used in this study can be made available to researchers collaborating with Appalachian Regional Healthcare under a research agreement. However, the data are not publicly available due to the need to preserve privacy of patient health information.
Abbreviations
BMI: body mass index; HF: heart failure; COVID-19: coronavirus disease 2019; ICU: intensive care unit; IRB: Institutional Review Board; LOS: length of stay
| References | ▴Top |
- COVID Live Update: 200,194,915 Cases and 4,257,832 Deaths from the Coronavirus - Worldometer [Internet]. [cited 2021 Aug 3]. Available from: https://www.worldometers.info/coronavirus/.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
doi - Association between cardiovascular risk factors and the severity of coronavirus disease 2019: nationwide epidemiological study in Korea.
- Abbasi J. The COVID heart-one year after SARS-CoV-2 infection, patients have an array of increased cardiovascular risks. JAMA. 2022;327(12):1113-1114.
doi pubmed - Bhatt AS, Jering KS, Vaduganathan M, Claggett BL, Cunningham JW, Rosenthal N, Signorovitch J, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Heart Fail. 2021;9(1):65-73.
doi pubmed - Chatrath N, Kaza N, Pabari PA, Fox K, Mayet J, Barton C, Cole GD, et al. The effect of concomitant COVID-19 infection on outcomes in patients hospitalized with heart failure. ESC Heart Fail. 2020;7(6):4443-4447.
doi pubmed - Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet. 2020;395(10232):1243-1244.
doi - Mujib M, Zhang Y, Feller MA, Ahmed A. Evidence of a "heart failure belt" in the southeastern United States. Am J Cardiol. 2011;107(6):935-937.
doi pubmed - Sisti N, Valente S, Mandoli GE, Santoro C, Sciaccaluga C, Franchi F, Cameli P, et al. COVID-19 in patients with heart failure: the new and the old epidemic. Postgrad Med J. 2021;97(1145):175-179.
doi pubmed - Mehra MR, Ruschitzka F. COVID-19 illness and heart failure: a missing link? JACC Heart Fail. 2020;8(6):512-514.
doi pubmed - Bader F, Manla Y, Atallah B, Starling RC. Heart failure and COVID-19. Heart Fail Rev. 2021;26(1):1-10.
doi pubmed - Ruge M, Gomez JMD, du Fay de Lavallaz J, Hlepas A, Rahman A, Patel P, Hoster C, et al. Impact of pre-existing heart failure on 60-day outcomes in patients hospitalized with COVID-19. Am Heart J Plus. 2021;4:100022.
doi pubmed - Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450-454.
doi pubmed - Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247-250.
doi pubmed - Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810.
doi pubmed - Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382(17):1653-1659.
doi pubmed - Job R, Abdul Qader M, Torres P, Al Abbasi B, Dewaswala N, Abdallah A, Chen K, et al. Renin-angiotensin system blocker in COVID-19. A single center study. J Cardiovasc Pharmacol. 2022;79(3):311-314.
doi pubmed - Hippisley-Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503-1511.
doi pubmed - Al Abbasi B, Torres P, Ramos-Tuarez F, Dewaswala N, Abdallah A, Chen K, Abdul Qader M, et al. Cardiac Troponin-I and COVID-19: a prognostic tool for in-hospital mortality. Cardiol Res. 2020;11(6):398-404.
doi pubmed - Manocha KK, Kirzner J, Ying X, Yeo I, Peltzer B, Ang B, Li HA, et al. Troponin and other biomarker levels and outcomes among patients hospitalized with COVID-19: derivation and validation of the HA2T2 COVID-19 mortality risk score. J Am Heart Assoc. 2021;10(6):e018477.
doi pubmed - Hana C, Aboulenain S, Dewaswala N, Narendran V. Does thrombocytopenia truly correlate with COVID-19 severity? Blood. 2020;136:39-40.
doi - Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr Clin Med. 2020;2(11):2048-2058.
doi pubmed - Stawinski PM, Dziadkowiec KN, Al-Abbasi B, Suarez L, Simms L, Dewaswala N, Torres P, et al. Model of End-Stage Liver Disease (MELD) score as a predictor of in-hospital mortality in patients with COVID-19: a novel approach to a classic scoring system. Cureus. 2021;13(5):e15179.
doi - Dziadkowiec KN, Stawinski PM, Dewaswala N, Suarez LSK, Simms LN, Gupta K, et al. S1133 MELD, MELD-Na, and MELD-albumin scores as predictors of mortality in patients with SARS-CoV-2: an uncanny relationship during troubling times. Off J Am Coll Gastroenterol ACG. 2020;115:S569.
doi - Suarez LS, Simms L, Dewaswala N, Gupta K, Dziadkowiec K, Stawinski P, et al. Liver function abnormalities and mortality in patients with coronavirus disease 2019 (COVID-19). Hepatology. 2020:285A.
- Dziadkowiec KN, Stawinski PM, Dewaswala N, Suarez LSK, Simms LN, Abbasi BA, et al. S1132 MELD score as a predictor of mortality in patients with SARS-CoV-2: a new application for a classic scoring system. Off J Am Coll Gastroenterol ACG. 2020;115:S568.
doi - Stawinski PM, Dziadkowiec KN, Dewaswala N, Suarez LSK, Simms LN, Abbasi BA, et al. S1021 MELD-Na score prognostic utility and implementation in clinically relevant outcomes of patients with SARS-CoV-2. Off J Am Coll Gastroenterol ACG. 2020;115:S521.
doi - Bhopalwala H, Dewaswala N, Kolagatla S, Wisnieski L, Piercy J, Bhopalwala A, Moka N. Predictors of mortality for patients with COVID-19 in the rural Appalachian region. Int J Gen Med. 2022;15:2207-2214.
doi pubmed - Dalia T, Lahan S, Ranka S, Acharya P, Gautam A, Goyal A, Mastoris I, et al. Impact of congestive heart failure and role of cardiac biomarkers in COVID-19 patients: A systematic review and meta-analysis. Indian Heart J. 2021;73(1):91-98.
doi pubmed - Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
doi pubmed - Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, Metra M. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22(6):957-966.
doi pubmed - Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133(9):1025-1031.
doi pubmed - Paleiron N, Mayet A, Marbac V, Perisse A, Barazzutti H, Brocq FX, Janvier F, et al. Impact of tobacco smoking on the risk of COVID-19: a large scale retrospective cohort study. Nicotine Tob Res. 2021;23(8):1398-1404.
doi pubmed - The role of nicotine in COVID-19 infection [Internet]. The Centre for Evidence-Based Medicine. [cited Jan 17, 2022]. Available from: https://www.cebm.net/covid-19/nicotine-replacement-therapy/.
- C SK, Kumar SA, Wei H. A computational insight of the improved nicotine binding with ACE2-SARS-CoV-2 complex with its clinical impact. ArXiv200414943 Q-Bio [Internet]. 2020 [cited Jan 17, 2022]; Available from: http://arxiv.org/abs/2004.14943.
- Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking. Nicotine Tob Res. 2020;22(9):1650-1652.
doi pubmed - COVID incidence, mortality rates remain much higher in rural areas [Internet]. University of Iowa College of Public Health. [cited Jan 8, 2022]. Available from: https://www.public-health.uiowa.edu/news-items/covid-incidence-mortality-rates-remain-much-higher-in-rural-areas/.
- Huang Q, Jackson S, Derakhshan S, Lee L, Pham E, Jackson A, Cutter SL. Urban-rural differences in COVID-19 exposures and outcomes in the South: A preliminary analysis of South Carolina. PLoS One. 2021;16(2):e0246548.
doi pubmed - Why COVID-19 vaccination rates are lower in rural areas of the U.S.? Syracuse University News. [Internet] 2021. [cited Jan 17, 2022] Available from: https://news.syr.edu/blog/2021/10/04/why-covid-19-vaccination-rates-are-lower-in-rural-areas-of-the-u-s/.
- Murthy BP. Disparities in COVID-19 vaccination coverage between urban and rural counties - United States, December 14, 2020 - April 10, 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2021;70. [cited Jan 17, 2022] Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7020e3.htm.
- Matthews KA, Croft JB, Liu Y, Lu H, Kanny D, Wheaton AG, Cunningham TJ, et al. Health-related behaviors by urban-rural county classification - United States, 2013. MMWR Surveill Summ. 2017;66(5):1-8.
doi pubmed - Dandachi D, Reece R, Wang EW, Nelson T, Rojas-Moreno C, Shoemaker DM. Treating COVID-19 in rural America. J Rural Health. 2021;37(1):205-206.
doi pubmed - Karim SA, Chen HF. Deaths from COVID-19 in rural, micropolitan, and metropolitan areas: a county-level comparison. J Rural Health. 2021;37(1):124-132.
doi pubmed - Remdesivir for the Treatment of Covid-19 - Final Report | NEJM [Internet]. [cited 2022 Mar 20]. Available from: https://www.nejm.org/doi/full/10.1056/nejmoa2007764.
- Reddy R, Chen K, Dewaswala N, Tuarez FR, Pino J, Martinez AC, et al. High incidence of spontaneous pneumothorax in critically ill patients with SARS-COV-2. Chest. 2020;158(4):A1191.
doi - Bhopalwala H, Mishra V, Do TV, Gudipati M, Ganti SS. COVID-19 infection and late manifestation of pulmonary aspergillosis. J Investig Med High Impact Case Rep. 2022;10:23247096211063332.
doi pubmed - Martinez AC, Dewaswala N, Tuarez FR, Pino J, Chait R, Chen K, et al. Validation of sofa score in critically ill patients with COVID-19. CHEST. 2020;158(4):A613.
doi - Aboulenain S, Dewaswala N, Ramos F, Torres P, Abdallah A, Qader MA, et al. The Effect of hydroxychloroquine on in-hospital mortality in COVID-19. HCA Healthc J Med [Internet]. 2020;1:20.
doi - Dexamethasone in hospitalized patients with COVID-19 | NEJM [Internet]. [cited Mar 20, 2022]. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2021436.
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384(9):795-807.
doi pubmed - DeFilippis EM, Reza N, Donald E, Givertz MM, Lindenfeld J, Jessup M. Considerations for heart failure care during the COVID-19 pandemic. JACC Heart Fail. 2020;8(8):681-691.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


