| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Case Report
Volume 13, Number 3, June 2022, pages 172-176
Resolution of Symptomatic Intermittent Sinoatrial Exit Block Associated With Unstable Angina Following Percutaneous Coronary Intervention
Kahtan Fadaha, c, Sandesh Yohannana, Juan Cartagenaa, Ruben Montanezb, Chanwit Roongsritongb
aDepartment of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX, USA
bDivision of Cardiovascular Medicine, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX, USA
cCorresponding Author: Kahtan Fadah, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX 79905, USA
Manuscript submitted April 20, 2022, accepted May 3, 2022, published online June 2, 2022
Short title: Blocking Lesion of SNA Leading to SA Exit Block
doi: https://doi.org/10.14740/cr1388
| Abstract | ▴Top |
Bradyarrhythmia commonly occurs because of degenerative fibrosis in the conductive system. Ischemic disease is a rare etiology and limited cases have demonstrated direct evidence of ischemia to the sinus node vessels. We report a 62-year-old Hispanic male with a significant medical history of diabetes mellitus type II (DM II), hypertension, and dyslipidemia who was admitted to our hospital for symptomatic sinoatrial (SA) exit block. Patient had no electrolyte abnormalities and our differential included ischemic vs. fibrotic or infiltrative pathologies, giving symptomatic bradycardia, cardiac chest pain, and high-risk factors for coronary artery disease. We decided to take him for cardiac catheterization which revealed sluggish, pulsatile flow into the SA nodal artery due to severe stenosis of the ostial right coronary along with sever distal left circumflex (LCX) lesion. The flow into the sinus nodal artery (SNA) markedly improved post percutaneous coronary intervention (PCI) of the right coronary artery (RCA) and distal LCX and restoration of flow into SNA. Resolution of his bradyarrhythmia and symptoms post intervention confirmed our suspicious for reversible ischemic sinus node dysfunctions. Therefore, ischemic pathologies should be thought of when other common etiologies are less likely. Coronary angiogram should be considered prior to pacemaker evaluation in these setting to avoid missing reversible causes of bradyarrhythmia.
Keywords: Reversible; Ischemia; Sinus nodal artery; Exit block; Percutaneous coronary intervention
| Introduction | ▴Top |
Sinus node dysfunction, or sick sinus syndrome, is the inability of the sinoatrial (SA) node to produce an adequate heart rate that meets the physiologic needs of the individual. Sinus node dysfunction includes sinus bradycardia, sinus pauses or arrest, chronotropic incompetence, failure of the return of SA nodal activity after electrical cardioversion, and sinus exit blocks [1]. Even though sinus node dysfunction presents as bradycardia, it can also present as tachycardia as part of tachycardia-bradycardia syndrome [2]. SA nodal exit block occurs because of interference with the delivery of impulses from the SA node to its neighboring atrial tissue.
The main causes of sinus node dysfunction can be differentiated into intrinsic (e.g., degenerative idiopathic fibrosis, cardiac remodeling, ischemia, etc.), or extrinsic (medications, metabolic abnormalities, or autonomic imbalances) [1]. Among these, advanced age causing secondary fibrosis and subsequent dysfunction of the SA node is the most common cause [3]. However, ischemic disease is another rare etiology of SA dysfunction. Only few cases have reported direct evidence of ischemia to the sinus node vessels. Here, we report symptomatic SA exit block with intermittent bradycardia secondary to severe stenosis of the ostial right coronary artery (RCA) and SA nodal artery slow flow.
| Case Report | ▴Top |
Investigations
The patient is a 62-year-old Hispanic male who presented with 4-week history of intermittent non-radiating retrosternal pressure associated with lightheadedness. The symptom was not related to exertion and usually resolved spontaneously. During these episodes, the patient would also notice a much slower heart rate than his baseline, at times as low as 30 beats per minute (bpm). His initial vital signs in the emergency department showed a heart rate of 39 bpm and blood pressure of 160/73 mm Hg. His heart rate shortly thereafter was reportedly normal without any intervention. Physical examination was otherwise unremarkable. The patient had a significant medical history of cardiovascular risk factors, including diabetes mellitus type II (DM II), hypertension, and dyslipidemia. He reported compliance on taking his medications of aspirin 81 mg daily, atorvastatin 20 mg daily, and metformin 500 mg twice daily. The patient acknowledged having a history of hypertension but denied any medication within the past year. He was not taking any heart rate-slowing medications. He reported a remote history of coronavirus disease 2019 (COVID-19) 10 months ago with mild symptoms. He denied a history of tobacco, alcohol, or any illicit drug abuse.
Diagnosis
Initial electrocardiogram (EKG) showed bradycardia with a pattern consistent with SA exit block (Fig. 1). His laboratory workup was unremarkable except for initial troponin T (TnT) level at 0.025 ng/mL (normal range 0.000 - 0.034 ng/mL). Our differentials consisted of fibrotic or infiltrative pathologies, electrolyte abnormalities such as hyperkalemia, and ischemic causes. But during initial lab evaluation electrolytes were within normal limits. The patient presented with symptomatic bradycardia, possible cardiac chest pain, and had multiple cardiovascular risk factors including diabetes mellitus, hypertension, and family history of coronary artery disease (CAD).
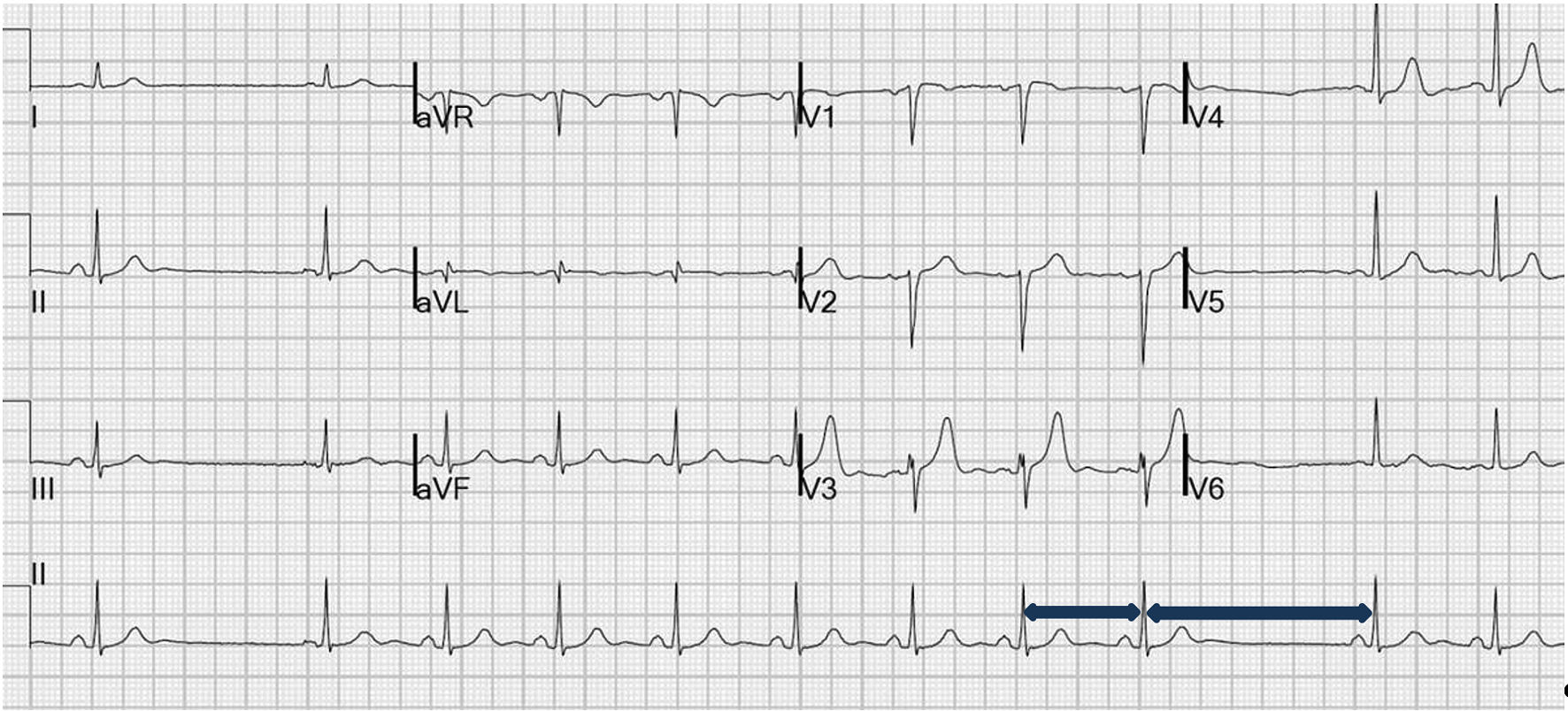 Click for large image | Figure 1. Electrocardiogram (EKG) showed sinus bradycardia with sinoatrial (SA) exit block, with blue arrow showing the length of R-R interval doubling with dropped P wave. |
The patient was diagnosed with unstable angina. He was admitted to the cardiac telemetry unit and was started on guideline-directed medical therapy for possible acute coronary syndrome except for beta-blocker or any other rate-slowing medications. The cardiac monitor overnight revealed intermittent sudden rate decrease consistent with SA exit block. TnT plateaued at 0.030 ng/mL within 12 h after the admission. Echocardiography showed normal left ventricular systolic and diastolic function with no regional wall motion abnormality or valvular dysfunction. He continued to report intermittent chest discomfort and lightheadedness.
Treatment
He subsequently underwent cardiac catheterization which revealed 90-95% ostial RCA stenosis proximal to sinus nodal artery (SNA) along with distal left circumflex (LCX) 90% lesion. The flow into the SNA was markedly slow and pulsatile (Fig. 2 and Supplementary Material 1, www.cardiologyres.org). Percutaneous coronary intervention (PCI) of the RCA and distal LCX was successfully performed resulting in normalization of the SNA flow (Fig. 3 and Supplementary Material 2, www.cardiologyres.org). Cardiac monitor for the following 36 h showed no further evidence of SA exit block. The patient also became asymptomatic with a heart rate averaging in the low 60s. He was discharged on aspirin 81 mg daily, clopidogrel 75 mg daily, and atorvastatin 80 mg daily.
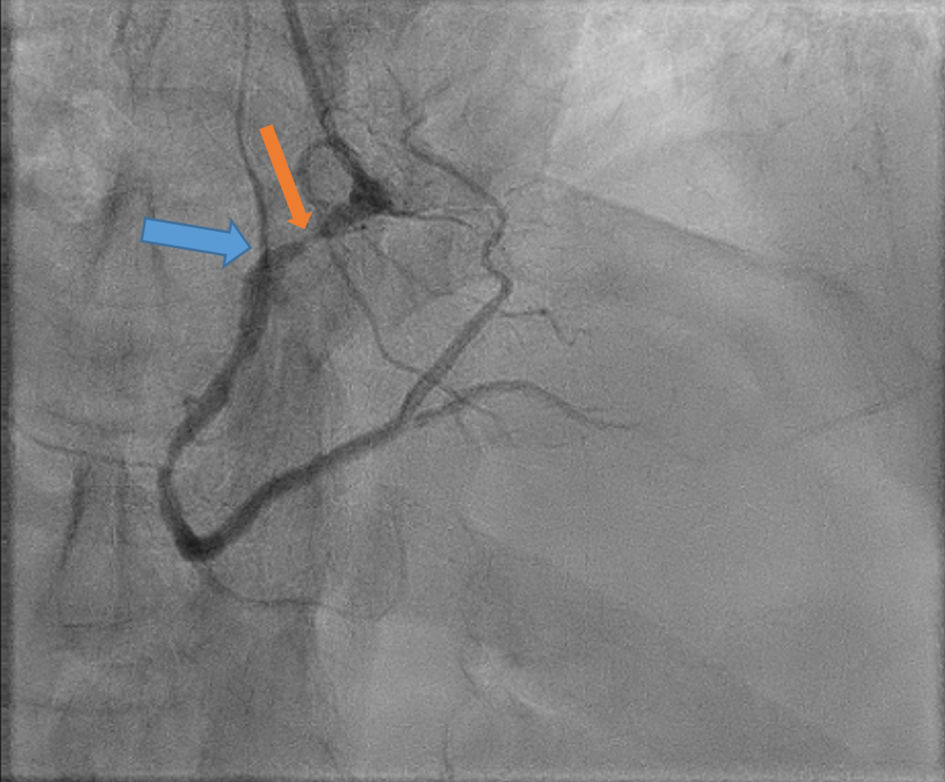 Click for large image | Figure 2. Angiogram showing sever ostial right coronary artery (RCA) stenosis proximal to sinus nodal artery (SNA) as showing in orange arrow, and markedly slow and pulsatile flow into the SNA (blue arrow). |
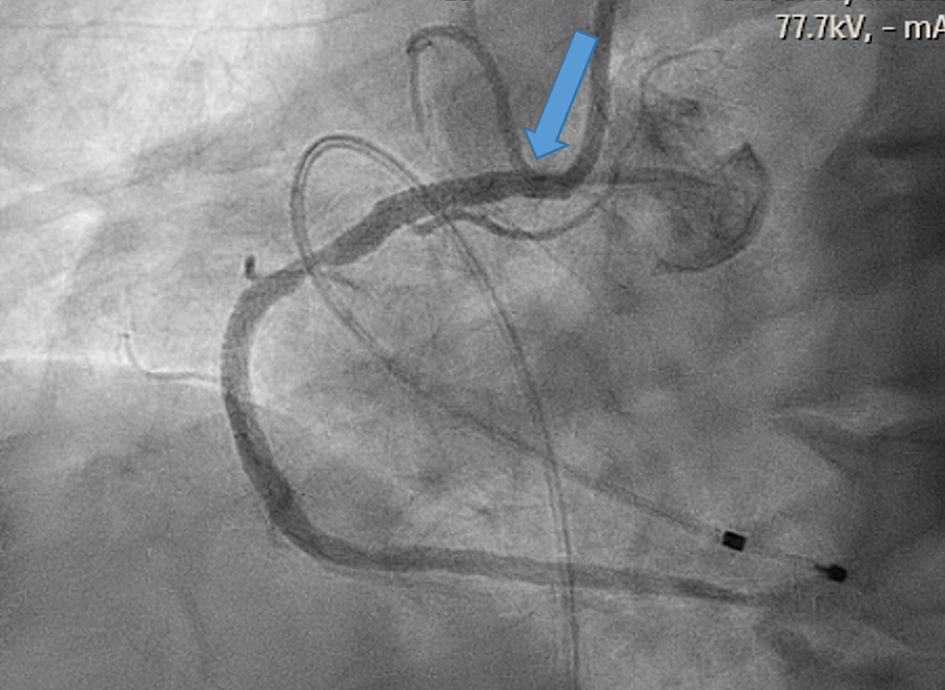 Click for large image | Figure 3. Angiogram showing restoration of flow into the right coronary artery (RCA) and sinus nodal artery (SNA) as showing in the blue arrow. |
Follow-up and outcomes
At 3-month follow-up, he continued to be asymptomatic with a stable heart rate of > 60 bpm. He was adherent to his optimal medical therapy and was asked to follow up in 6 months.
| Discussion | ▴Top |
The SA node, a complex compact region in the junction of superior vena cava and the right atrium, is the dominant pacemaker of the heart. The SA node depolarizes and produces action potential almost synchronously [1]. The most common cause of bradyarrhythmia is thought to be degenerative fibrosis of sinus node and atrioventricular (AV) node dysfunction. Ischemic disease is a rare etiology of sinus node dysfunction.
SA node has a complex blood supply. The SA nodal artery that supplies it can arise from either right or left coronary artery. There is no relationship between the dominant coronary circulation of the heart and the SA nodal artery origin. In 63% of the case the SA nodal artery originates from the RCA. When it arises from the left coronary artery it usually does so from the proximal portion of the left circumflex branch [2]. In our patient the SA nodal artery originated from the RCA.
Sinus node dysfunction, or sick sinus syndrome, is the inability of the SA node to produce an adequate heart rate that meets the physiologic needs of the individual. Sinus node dysfunction includes sinus bradycardia, sinus pauses or arrest, chronotropic incompetence, failure of the return of SA nodal activity after electrical cardioversion, and sinus exit blocks [3]. Even though sinus node dysfunction presents as bradycardia, it can also present as tachycardia as part of tachycardia-bradycardia syndrome [4]. SA nodal exit block occurs because of interference with the delivery of impulses from the SA node to its neighboring atrial tissue.
The main causes of sinus node dysfunction can be differentiated into intrinsic (e.g., degenerative idiopathic fibrosis, cardiac remodeling, ischemia, etc.), or extrinsic (medications, metabolic abnormalities, or autonomic imbalances) [3]. Among these, advanced age causing secondary fibrosis and subsequent dysfunction of the SA node is the most common cause [5]. Myocardial ischemia which is caused by the imbalance between myocardial oxygen supply and demand has also been shown to be a cause of sinus node dysfunction especially when the blood supply to the sinus node itself is compromised. Kyriakidis et al found asymptomatic sinus node dysfunction in patient with inferior myocardial infarction and one-vessel disease when there is occlusion of the infarct-related coronary artery proximal to the site of origin of the sinus node artery [6]. Additionally, SA exit block resulting from an iatrogenic transient reduction in SA nodal artery flow following stent implantation in the proximal RCA has also been reported [7].
The incidence of sinus node dysfunction increases with age and was also found to be higher in those with greater body mass index, lower heart rate, and those with coexisting hypertension, right bundle branch block, and other cardiovascular diseases [4]. Patient with sinus node dysfunction usually presents with symptoms like dizziness, palpitations, exertional dyspnea, easy fatigability, angina, or syncope [4, 8].
Herein, we described a relatively young patient who presented with an SA exit block associated with chest pain and normal cardiac biomarker consistent with unstable angina. He was not taking any heart rate-slowing medication and had no metabolic derangement on presentation. Lack of obvious signs of myocardial ischemia on EKG has been previously reported by Patel et al, which can be explained by higher prevalence of collaterals in patients presented with bradycardia than those presented with tachycardia [9]. In our patient, sluggish and pulsatile flow into SA nodal artery was noted on the initial angiogram and the SA exit block in patient also resolved almost immediately after PCI.
In a similar case report as reported by Kumar et al, the patient who presented with syncopal episode was found to have a junctional rhythm. The patient who subsequently underwent a cardiac catheterization was found to have mid-RCA total occlusion with a narrowed SA nodal branch. Post PCI the patient apparently remained in sinus rhythm [10].
Even though SNA occlusion does cause SA nodal dysfunction as evident from the SA nodal dysfunction that was demonstrated because of post-PCI occlusion of the RCA as described by Koren et al [11], Kyriakidis et al [7], and Haraki el al [12], establishing a definite causal effect of ischemia on sinus nodal conduction disturbances is sometimes difficult in chronic CAD. Even though CAD can cause bradyarrhythmia, in one study where patients who presented with symptomatic bradyarrhythmia and who either had either a sinus node dysfunction or AV block the incidence of CAD was 20%. However, in a similar study, the incidence was as high as 60% [13, 14]. But these differences in the incidence of CAD in patients with bradyarrhythmia can be attributed to the baseline difference in the characteristics of the population in the two studies. Presence of multiple cardiovascular risk factors in the patient with bradyarrhythmia correlated better with the presence of a CAD [15]. Our patient who had a bradyarrhythmia underwent a coronary angiogram given the multiple cardiovascular high-risk factors he was having.
Published reports on the effect of coronary revascularization on presumably ischemia-related conduction disturbance reversal so far have mostly been limited to AV nodal dysfunction in acute ischemic settings. Ramamurthy et al in their case report of a patient with acute inferior myocardial infarction who presented with complete heart block treated with successful PCI, which was effective in reversing persistent complete heart block, thus avoiding implantation of a permanent pacemaker, suggested that PCI may be considered as a treatment option before recommending permanent pacemaker, especially for complete heart block [16]. Studies comparing the impact of PCI on the reversal of the conduction defects have been however equivocal. In his study Zhong et al demonstrated that patients with asymptomatic bradyarrhythmia who had a CAD and underwent PCI delayed the timing for pacemaker implantation and the aggravation rate of arrhythmias in the first 2 years of follow-up [17]. But Yesil et al found coronary revascularization to have very little, if any, impact on returning of normal AV conduction, which can be attributed to the fact that damage to the conduction system is irreversible [18]. We believe that the effect of coronary revascularization on acute ischemia-related sinus nodal dysfunction, as described herein has not been studied. In these patients where a permanent damage to the conduction system has not ensued may benefit from early PCI. Hence, PCI should be considered as an option before recommending permanent pacemaker implantation as the sinus node dysfunction may be reversible.
Conclusions
We described a case of severe symptomatic bradycardia secondary to SA exit block associated with unstable angina. His cardiac catheterization revealed subtotal occlusion in the ostial RCA with sluggish and pulsatile flow into the SNA along with stenosis in the distal LCX. The SA exit block and his symptoms resolved after PCI of both lesions. Our case illustrated that in certain clinical scenarios, reversible ischemic causes should be considered in a relatively young patient who presents with symptomatic bradycardia. In these patients, reversal of the SA nodal artery flow can lead to a complete recovery of the sinus node function.
Learning points
Learning points from this case report included: 1) Considering ischemic pathologies in the setting of symptomatic intermittent bradycardia in the appropriate population; 2) Reversible causes of bradyarrhythmia such as RCA occlusion and slow flow in SNA should be addressed before permanent pacemaker placement.
| Supplementary Material | ▴Top |
Suppl 1. Video 1 showing the flow into the sinus nodal artery (SNA) was markedly slow and pulsatile prior to percutaneous coronary intervention (PCI).
Suppl 2. Video 2 showing improved flow into the sinus nodal artery (SNA) and right coronary artery (RCA) after percutaneous coronary intervention (PCI) of the RCA.
Acknowledgments
None to declare.
Financial Disclosure
There is no funding for this work.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Dr. Fadah formulated the paper, did the initial research of the topic, wrote abstract, case presentation, learning points and reviewed the entire document. Dr. Yohannan and Cartagena wrote the introduction and discussion. They also provided rest of reference in this document. Dr. Montanez provided all clinical images, videos, and reviewed the document and provided comments and suggestions. Dr. Roongsritong guided the team in how to structure the paper, edited/revised statements when needed, provided clear instructions for presenting the case report.
Data Availability
The authors declare that data supporting the finding of this study are available within the article.
Abbreviations
SA: sinoatrial; COVID-19: coronavirus disease 2019; EKG: electrocardiogram; TnT: troponin T; RCA: right coronary artery; SNA: sinus nodal artery; LCX: left circumflex; PCI: percutaneous coronary intervention; CAD: coronary artery disease
| References | ▴Top |
- Ferrer MI. The sick sinus syndrome in atrial disease. JAMA. 1968;206(3):645-646.
doi pubmed - Jensen PN, Gronroos NN, Chen LY, Folsom AR, deFilippi C, Heckbert SR, Alonso A. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64(6):531-538.
doi pubmed - Hatle L, Bathen J, Rokseth R. Sinoatrial disease in acute myocardial infarction. Long-term prognosis. Br Heart J. 1976;38(4):410-414.
doi pubmed - Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47(4):658-687.
doi - Pejkovic B, Krajnc I, Anderhuber F, Kosutic D. Anatomical aspects of the arterial blood supply to the sinoatrial and atrioventricular nodes of the human heart. J Int Med Res. 2008;36(4):691-698.
doi pubmed - Kyriakidis M, Trikas A, Triposkiadis F, Kofinas G, Tsakiris M, Antonopoulos A, Gialafos J, et al. Sinus node dysfunction in acute inferior myocardial infarction. Role of sinus node artery and clinical course in patients with one-vessel coronary artery disease. Cardiology. 1997;88(2):166-169.
doi pubmed - Kyriakidis MK, Kourouklis CB, Papaioannou JT, Christakos SG, Spanos GP, Avgoustakis DG. Sinus node coronary arteries studied with angiography. Am J Cardiol. 1983;51(5):749-750.
doi - Ferrer MI. The sick sinus syndrome. Circulation. 1973;47(3):635-641.
doi pubmed - Patel SR, Breall JA, Diver DJ, Gersh BJ, Levy AP. Bradycardia is associated with development of coronary collateral vessels in humans. Coron Artery Dis. 2000;11(6):467-472.
doi pubmed - Kumar D, Pawar A, Sabnis G, Shah H, Lanewar C, Kerkar P, More D. Reversible SA nodal dysfunction. Interv Cardiol. 2018;10(2):37-38.
- Koren O, Antonelli D, Khamaise R, Ehrenberg S, Rozner E, Turgeman Y. Sinus node dysfunction due to occlusion of the sinus node artery during percutaneous coronary intervention. J Interv Cardiol. 2021;2021:8810484.
doi pubmed - Haraki T, Hirase H, Hoda S, Hashimoto M, Higashi M. Sinus dysfunction after stent implantation in the right coronary artery immediately recovered after reflow in the sinus node artery. Cardiovasc Interv Ther. 2014;29(2):173-176.
doi pubmed - Hsueh CW, Lee WL, Chen YT, Ting CT. The incidence of coronary artery disease in patients with symptomatic bradyarrhythmias. Jpn Heart J. 2001;42(4):417-423.
doi pubmed - Brueck M, Bandorski D, Kramer W. Incidence of coronary artery disease and necessity of revascularization in symptomatic patients requiring permanent pacemaker implantation. Med Klin (Munich). 2008;103(12):827-830.
doi pubmed - Vyas P, Meghnathi H, Joshi H, Brahmbhatt J, Dake R, Satpute A, Patel K. Coexistent coronary artery disease in Indian patients undergoing permanent pacemaker implantation (PPI) for symptomatic bradyarrhythmia. Indian Heart J. 2021;73(5):577-581.
doi pubmed - Ramamurthy S, Anandaraja S, Matthew N. Percutaneous coronary intervention for persistent complete heart block complicating inferior myocardial infarction. J Invasive Cardiol. 2007;19(12):E372-374.
- Zhong L, Gao Y, Xia H, Li X, Wei S. Percutaneous coronary intervention delays pacemaker implantation in coronary artery disease patients with established bradyarrhythmias. Exp Clin Cardiol. 2013;18(1):17-21.
- Yesil M, Bayata S, Arikan E, Yilmaz R, Postaci N. Should we revascularize before implanting a pacemaker? Clin Cardiol. 2008;31(10):498-501.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


