| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 12, Number 4, August 2021, pages 238-243
Global and Regional Longitudinal Strain Reduction in Breast Cancer Patients After First Chemotherapy Cycle With Fluorouracil, Adriamycin, and Cyclophosphamide Regimen
Astri Astutia, c, Erwinanto Erwinantoa, Muhammad Rizki Akbara, Erwan Martantoa, Dharmayanti Fransisca Badudub
aDepartment of Cardiology and Vascular Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Jl. Pasteur No. 38, Bandung 40161, Indonesia
bDepartment of Surgery, Oncology Division, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Dr. Hasan Sadikin General Hospital, Jl. Pasteur No. 38, Bandung 40161, Indonesia
cCorresponding Author: Astri Astuti, Department of Cardiology and Vascular Medicine, Universitas Padjadjaran, Dr. Hasan Sadikin General Hospital, Jl. Pasteur No. 38, Bandung 40161, Indonesia
Manuscript submitted January 25, 2021, accepted May 4, 2021, published online July 9, 2021
Short title: GLS and RLS After First FAC in Breast Cancer
doi: https://doi.org/10.14740/cr1229
| Abstract | ▴Top |
Background: Chemotherapy with fluorouracil, adriamycin, and cyclophosphamide (FAC) regimen in breast cancer patients may cause myocardial injury and necrosis, thereby attenuating global and regional longitudinal strain (GLS and RLS). It is unclear whether the first chemotherapy cycle would cause GLS and RLS reduction and which segment would be most affected by the chemotherapy. The purpose of the study was to investigate the effect of the first chemotherapy cycle on GLS and RLS reduction.
Methods: This was a prospective single-center cohort study of patients with breast cancer who underwent the first chemotherapy cycle with a FAC regiment. The GLS and RLS were measured using speckle tracking echocardiography and left ventricular ejection fraction (LVEF) measured with Simpson’s biplane. The echocardiography was performed before and 3 weeks after the first chemotherapy cycle. We compared the value of GLS, RLS, and LVEF before and after chemotherapy using paired t-test analysis.
Results: Thirty-six breast cancer patients were enrolled in the study. The GLS and RLS were reduced significantly at 3 weeks compared to baseline. The RLS of the basal anteroseptal, basal anterolateral, mid anterolateral, mid inferolateral, and all apical segments declined significantly from baseline. The largest RLS decline was detected in the apicoanterior segment. The post-chemotherapy GLS but not LVEF was significantly lower than that before treatment.
Conclusion: The GLS and RLS of patients who underwent first cycle chemotherapy with FAC declined significantly than that before treatment, especially at the apicoanterior segment. LVEF was not altered after first cycle chemotherapy.
Keywords: Global longitudinal strain; Regional longitudinal strain; Breast cancer; Fluorouracil, adriamycin, and cyclophosphamide regimen; Cardiotoxicity
| Introduction | ▴Top |
Breast cancer is the most prevalent cancer globally, which accounts for almost a quarter of cancer cases in women [1] and is the second most common cancer in Indonesia after cervical cancer [2]. The survival rate of breast cancer patients increases every year, due to improvements in early detection and treatment [3]. Unfortunately, chemotherapy using anthracycline-contained regimen, such as fluorouracil, adriamycin, and cyclophosphamide (FAC) as the first-line treatment, can cause fatal complications such as cardiotoxicity [4].
Chemotherapy with the FAC regiment can lead to injury and necrosis of the myocardium, which will decrease subclinical left ventricular systolic function, assessed by global longitudinal strain (GLS) and regional longitudinal strain (RLS) [5]. Early detection of subclinical left ventricular dysfunction followed by administration of cardioprotective agents can prevent and decrease the incidence of cardiotoxicity and improve the reversibility of left ventricular function if cardiotoxicity has occurred [6]. Early detection and administration of cardioprotective agents also can reduce morbidity and mortality due to cardiotoxicity [3]. The purposes of the study were to investigate the effect of the first chemotherapy cycle on the GLS and RLS reduction, and also to evaluate the most affected segments by the chemotherapy.
| Materials and Methods | ▴Top |
The study was a prospective cohort, pre-, and post-study, conducted from October to November 2016 at Hasan Sadikin General Hospital, Bandung, Indonesia. The Institutional Review Board of Hasan Sadikin General Hospital, Bandung has approved this study and all participant have signed the written informed consent. This study was conducted in concordance with the ethical standards on human subjects of the Helsinki Declaration.
We studied breast cancer patients over 18 years old who indicated to undergo chemotherapy with the FAC regimen, either adjuvant or neoadjuvant, and had signed an informed consent letter to participate in the study. The patients were followed up at 3 weeks after the first cycle and reviewed for echocardiography.
The exclusion criteria before chemotherapy were acute and chronic heart failure, poor echocardiography window, valvular heart disease, congenital heart disease, heart rhythm other than sinus rhythm, pulmonary embolism, and chronic kidney disease. Exclusion criteria during or after the first chemotherapy cycle were acute coronary syndromes (ACS), pulmonary embolism, hypertensive emergency, stroke, and acute kidney injury. All subjects had undergone the first chemotherapy cycle with the FAC regimen, consisting of fluorouracil 600 mg/m2 IV, adriamycin 60 mg/m2 IV, and cyclophosphamide 600 mg/m2 IV. The adriamycin given was doxorubicin hydrochloride, and no one received liposomal adriamycin. None of the patient received cardio-protectors such as beta-blockers nor angiotensin-converting enzyme inhibitor to prevent cardiotoxicity.
Research procedure
We gathered the clinical history, and echocardiography parameters such as the subclinical left ventricular function, described by GLS, RLS, and left ventricular ejection fraction (LVEF) at baseline, around a month before chemotherapy initiation, and 3 weeks after the first cycle was completed, right before the second cycle.
Measurement
GLS and RLS
The GLS and RLS was assessed by speckle tracking echocardiography techniques (unit, %). We recorded five cardiac cycles in 2D grayscale images with 50 - 80 frames per second and evaluated at the end of the systole phase (right after the aortic valve closure). General Electric (GE) Vivid 7 and T-8 echocardiography was utilized, and then the data were analyzed offline through the ECHOPAC software version of 113. Two echocardiography consultant cardiologists interpreted the GLS and RLS, and then an interobserver variability test was examined to determine its reliability at our hospital.
Statistical analysis
The analysis of baseline characteristics, including demography, cardiovascular risk factors, and cancer type, was presented as mean and standard deviation in normally distributed numeric variables, median and range in not normal ones, and percentages for the categoric. The normality of the numeric variables was tested using the Shapiro-Wilks. Comparisons of GLS, RLS, and LVEF values before and after chemotherapy were performed using paired t-test analysis. Statistical calculations were aided by an IBM software for Statistical Analysis Software Package (SPSS) version 16 for Windows operating systems. All statistical tests were performed at the 5% significance level.
| Results | ▴Top |
Baseline characteristics
Fifty-six breast cancer patients met the inclusion criteria and 20 of them were excluded due to poor echocardiography window. The review of the subjects’ enrolment can be seen in Figure 1. All of the patients were women and almost everyone had invasive ductal carcinoma mammae and underwent adjuvant chemotherapy. The average age was 46 ± 9 years old and most of them were overweight. The most prevalent cardiovascular disease (CVD) risk factors were obesity (33%), followed by hypertension (8%) and hypertensive heart disease (8%). None of them had left ventricular dysfunction at baseline (LVEF > 53%), but four patients had baseline GLS less than -18%. The mean RLS at baseline was less than -18% at base anterior, anteroseptal, inferoseptal, inferior, and inferolateral. The baseline characteristics are listed in Table 1. Patients were monitored at 3 weeks after the first chemotherapy cycle and nobody was excluded at the end of the follow-up.
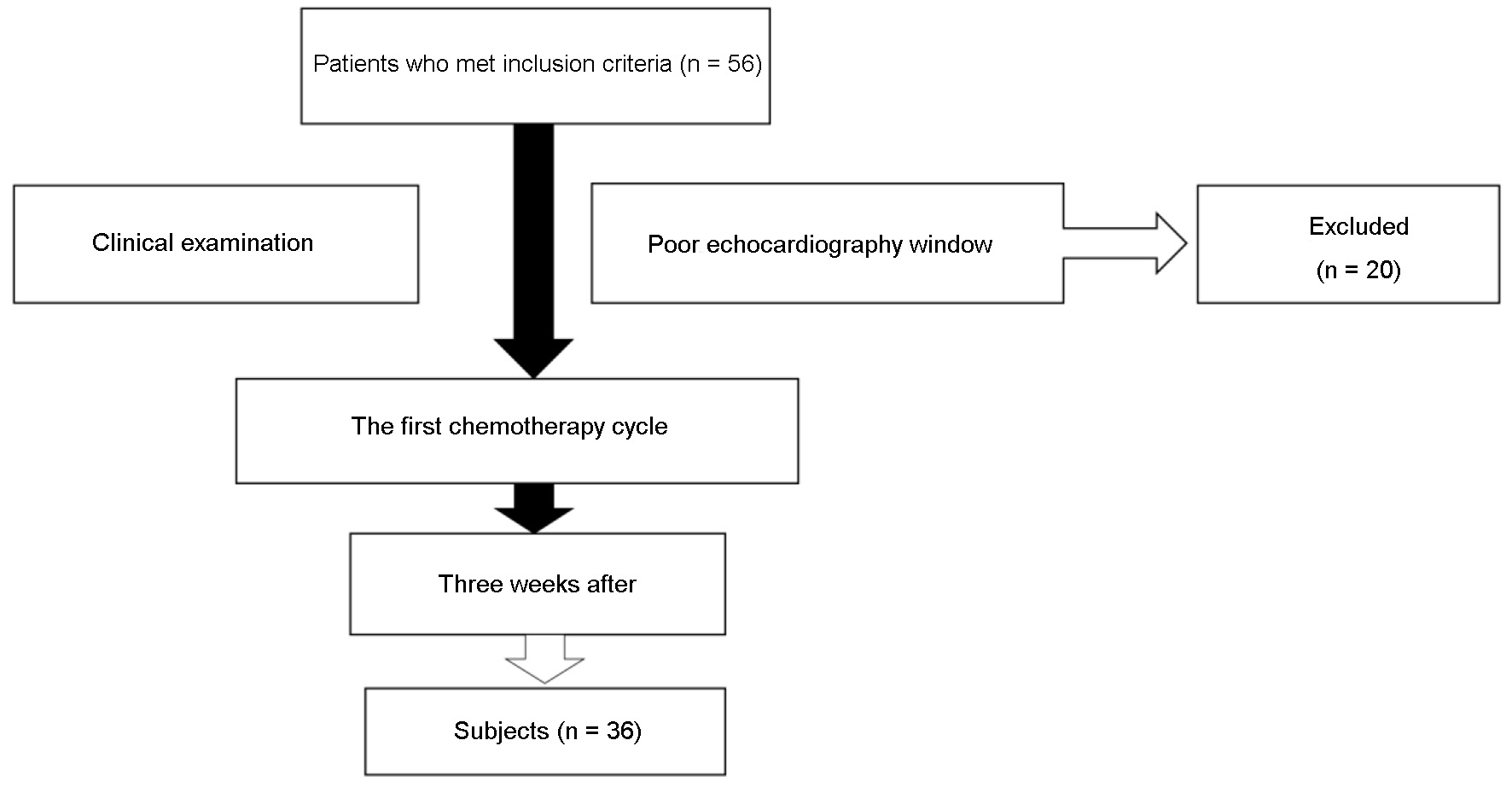 Click for large image | Figure 1. Subjects enrolment and assortment. |
 Click to view | Table 1. Baseline Characteristics |
Subclinical left ventricular dysfunctions, defined by GLS absolute number less than -18% or 15% reduction of GLS, were already seen in two-thirds of the patients. The GLS was reduced to below -18% in 11 patients (30%) and reduced 15% in also 11 of them (30%). In total, there were 40.7% of the patients having GLS reduction based on both criteria. No subjects were excluded after the first cycle.
The RLS was significantly reduced at base inferoseptal, anterolateral, mid inferolateral, anterolateral, apicoanterior, apicoseptal, apicolateral, apicoinferior, and apical cap segments. Even though some segments also reduced non-significantly to below -18%. The involved segments are shown in Figure 2.
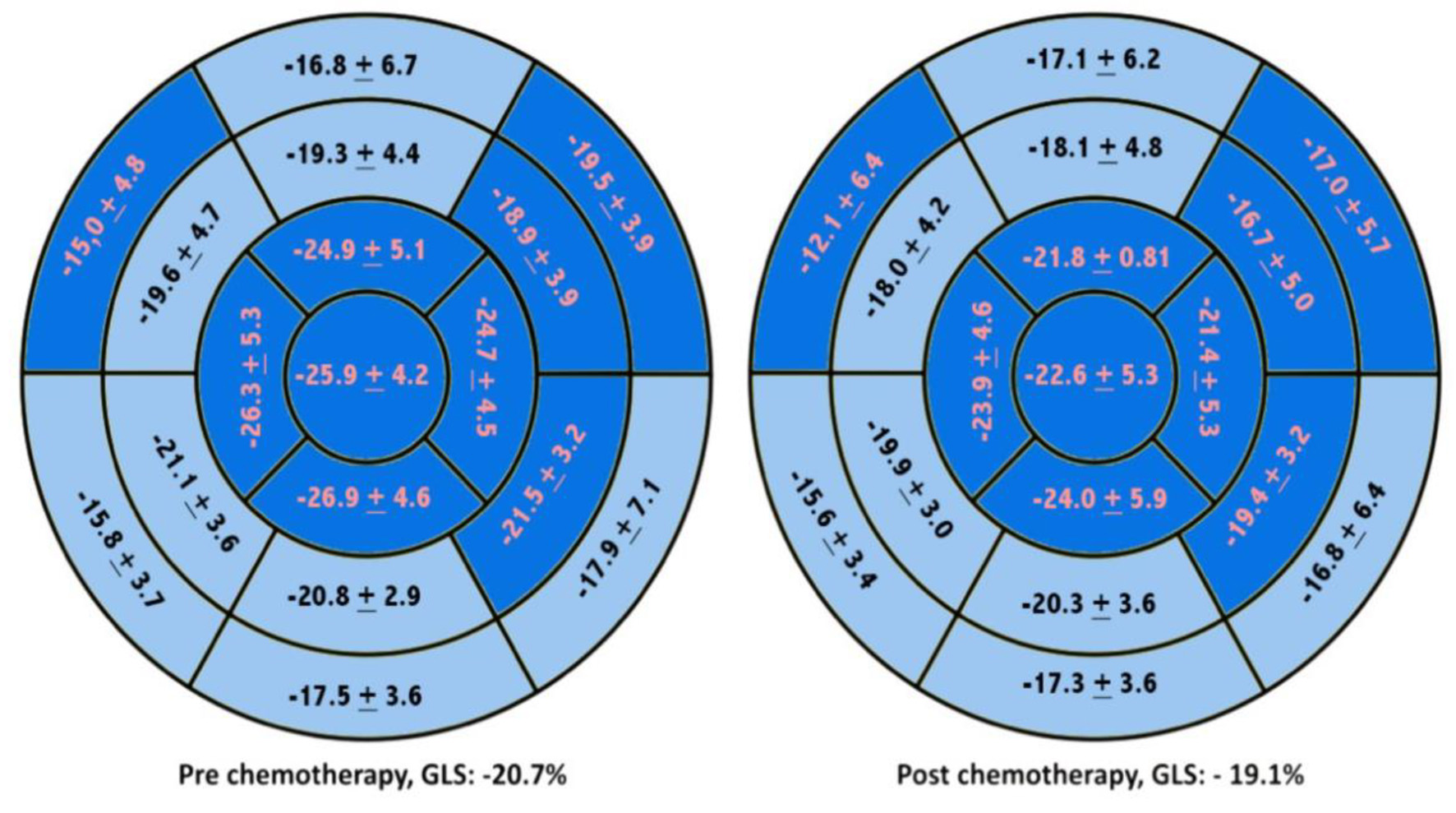 Click for large image | Figure 2. Worsening of RLS during chemotherapy with anthracyclines regiment. The segments shown in dark blue represented significant worsening of RLS by the t-test. RLS: regional longitudinal strain. |
Interobserver variability
The average difference between the first and second analysts based on Bland-Altman analysis was less than 10%. This means GLS and RLS examinations conducted at our center were consistent and trustworthy. Figure 3 shows the Bland-Altman study.
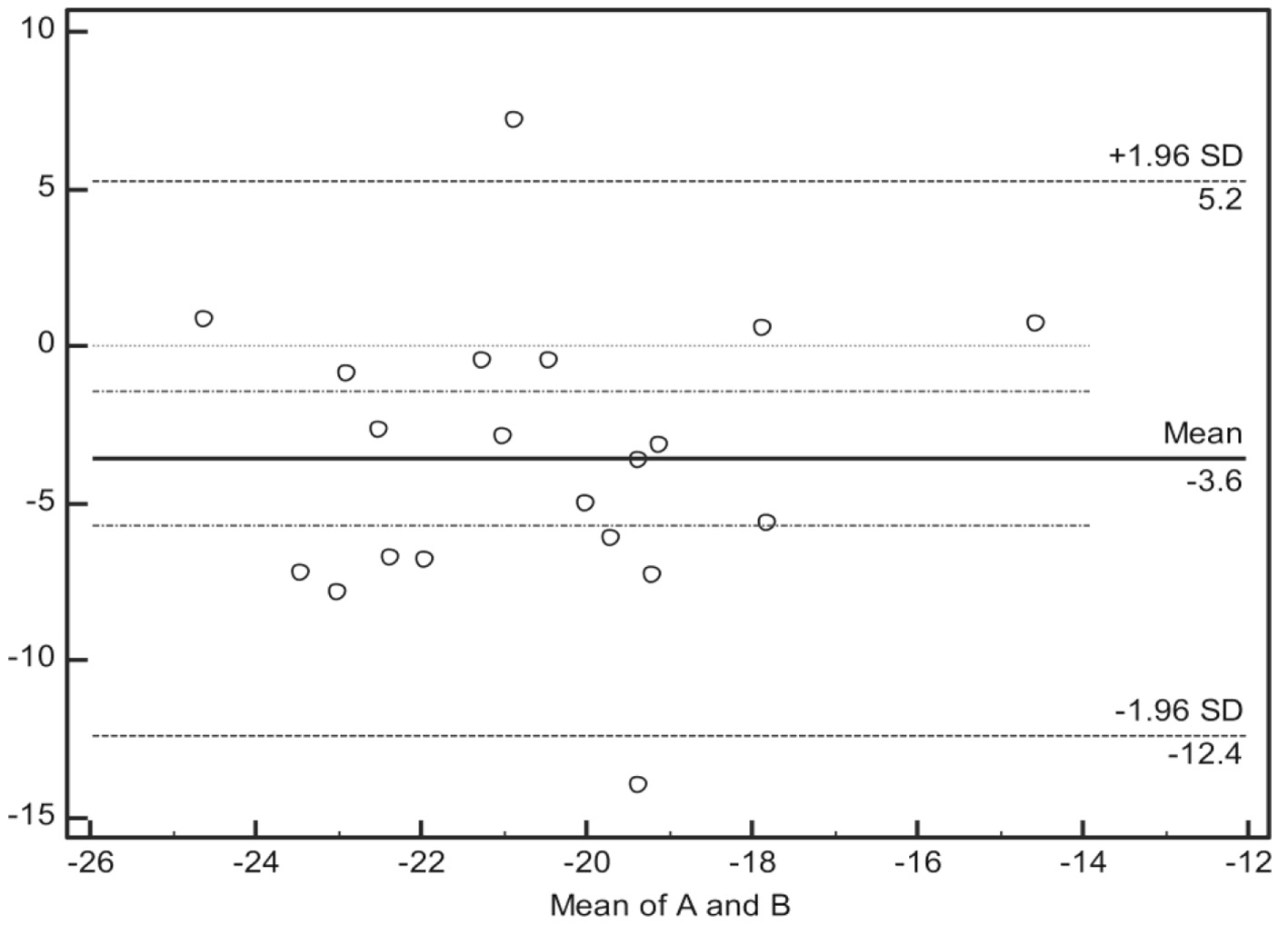 Click for large image | Figure 3. The Bland-Altman analysis showing that the difference in GLS value was less than 10% in our center. GLS: global longitudinal strain. |
GLS and RLS
Based on the t-test analysis, the GLS declined significantly at 3 weeks after chemotherapy (-20.7±2.4% and -19.1±2.8%, average reduction -1.63±2.83%, P < 0.05). Whereas the LVEF reduction was not significant (67.8±4.5% and 65.8±5.1%, average reduction 2.0% (-0.2-4.3%), P > 0.05). Table 2 shows the comparison of GLS and LVEF biplane reduction.
 Click to view | Table 2. Comparative Study of GLS and LVEF at Baseline and 3 Weeks After the First Chemotherapy Cycle Using T-Test |
The RLS of the basal anteroseptal, basal anterolateral, mid anterolateral, mid inferolateral, and all apical segments (P < 0.05) was significantly reduced. The most significant decline was found in apicoanterior segment (-24.9±5.1% and -21±8.1%, mean 3.9% (1.3-6.5%); P < 0.05). The details of the RLS alterations are shown in Table 3.
 Click to view | Table 3. Comparison of the RLS Value at Baseline and 3 Weeks After the First Chemotherapy Cycle Using T-Test Analysis |
Basal and mid anterolateral segments were reduced to below -18% and statistically significant, while the apicoanterior segment had the most significant decline of GLS absolute value, but still within a normal range.
| Discussion | ▴Top |
Awareness of cardiotoxicity due to anthracycline chemotherapy has increased over time because it is preventable by earlier detection and cardio-protective initiation. Therefore, detection of subclinical left ventricular dysfunction is crucial to ascertain the appropriate timing for prevention commencement. Initial reduction of GLS can predict the advancement of cardiotoxicity in the forthcoming [3, 5].
Several studies revealed that GLS less than -18% [7] or 10-14% GLS reduction can better predict cardiotoxicity [5, 8-12]. The ACC/AHA consensus determined that myocardial dysfunction already occurred at GLS less than -18%. While, the ESC position paper agreed that 15% GLS reduction from baseline is considered as subclinical dysfunction, and has a tendency to become cardiotoxic cardiomyopathy [4, 13].
The essential findings in our study are the first chemotherapy cycle is already reducing GLS and RLS at 3 weeks afterward. A significant reduction of GLS, through which the absolute value was still within a normal range, was -1.63±2.83%. Previous studies showed an average GLS reduction between -1.5% at 1 week after completion of chemotherapy and -2.03% at 6 weeks after chemotherapy [5]. Other studies have shown that the GLS decreased after 1 - 3 months after the chemotherapy initiation and continually declined to its completion.
The mean GLS decline was 8% compared to baseline; however, almost half of the patients had subclinical left ventricular dysfunction [13], which even can be considered as mild cardiotoxic using current criteria [14]. This is quite a large number, considering that it occurred after receiving very low dose anthracycline (60 mg/m2). Other studies usually had GLS reduction at a cumulative anthracycline dose of 240 mg/m2 [3, 4]. The lower amount and a high number of subclinical left ventricular dysfunction or mild cardiotoxicity in our study encourage us to consider whether the echocardiography should be performed after each cycle.
The RLS was also reduced 3 weeks after the first chemotherapy cycle, even though most of the absolute value moreover was within the normal limit. Eight of the 17 segments were decreased significantly with the most reduction occurring at apicoanterior segment. However, the basal and mid anterolateral segments were significantly reduced to lower than -18%, thus reflecting any subclinical deterioration. A different result was observed compared to earlier studies that showed septal and anterior segments were most affected by chemotherapy [8, 15]; hence, it suggested that probably no significant predilection segments complicated.
The LVEF determined cardiotoxicity due to chemotherapy occurrence, and its value was also reduced in our study, but not significant. No one had cardiotoxicity after the first cycle (LVEF reductions > 10% to an absolute value of LVEF to < 53%) due to a very low dose of anthracycline. The result was in line with former reports that showed the inclination of cardiotoxicity incidence with cumulative dose. A previous study showed that cardiotoxicity can occur at 3, 6 months, and a year after [4].
Even though recent reports and international position paper endorsed speckle tracking echocardiography technique to assist cardiotoxicity prevention [4], the utilization of the method is still sparse in our daily procedure. The outcomes of our study are aligned with prior studies and emphasize the usage of longitudinal strain as early as possible, perhaps after each cycle, to evaluate for subclinical left ventricular dysfunction and recognize the cardiotoxicity onset.
Some limitations in our study were a relatively small number of patients due to a pilot study. We also did not conduct a long-term follow-up, thus we cannot see the prevalence of cardiotoxicity and validate the used cutoff.
Conclusion
The first chemotherapy cycle has already resulted in GLS and RLS reduction, especially in the apicoanterior, basal, and mid anterolateral segments, however, not the LVEF. The GLS and RLS are necessary examinations in breast cancer patients during chemotherapy as an early detection to prevent cardiotoxicity occurrence.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The participants signed the written informed consent.
Author Contributions
Astri Astuti contributed to conception and design of the study, collection, analysis and interpretation of the data, writing and revision of the manuscript. Erwinanto Erwinanto contributed to design of the study, analysis and interpretation of the data, and reviewing and editing of the manuscript. Muhammad Rizki Akbar contributed to design of the study, collection, analysis and interpretation of the data, reviewing and editing of the manuscript. Erwan Martanto contributed to design of this study, collection, analysis and interpretation of the data, reviewing and editing of the manuscript. Dharmayanti Fransisca Badudu contributed to design of the study, reviewing and editing of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953.
doi pubmed - Tim Riskesdas 2018. Laporan nasional riset kesehatan dasar 2018. Laporan Nasional Riset Kesehatan Dasar 2018. Jakarta. 2018.
- Cardinale D, Lenihan DJ, Gheorghiade M, Baer L, Hamo CE, Nohria A, Butler J, et al. Cancer therapy-related cardiac dysfunction and heart failure part 1: definitions, pathophysiology, risk factors, and imaging. Circ Hear Fail. 2016;9(1):1-21.
doi - Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768-2801.
doi pubmed - Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25 Pt A):2751-2768.
doi pubmed - Jagsi R, Jiang J, Momoh AO, Alderman A, Giordano SH, Buchholz TA, Pierce LJ, et al. Cancer therapy-related cardiac dysfunction and heart failure part 2: prevention, treatment, guidelines, and future directions. Circ Hear Fail. 2017;263(2):219-227.
- Oikonomou EK, Kokkinidis DG, Kampaktsis PN, Amir EA, Marwick TH, Gupta D, Thavendiranathan P. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4(10):1007-1018.
doi pubmed - Stoodley PW, Richards DA, Hui R, Boyd A, Harnett PR, Meikle SR, Clarke J, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011;12(12):945-952.
doi pubmed - Arciniegas Calle MC, Sandhu NP, Xia H, Cha SS, Pellikka PA, Ye Z, Herrmann J, et al. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer. 2018;18(1):1037.
doi pubmed - Mornos C, Petrescu L. Early detection of anthracycline-mediated cardiotoxicity: the value of considering both global longitudinal left ventricular strain and twist. Can J Physiol Pharmacol. 2013;91(8):601-607.
doi pubmed - Kang Y, Cheng L, Li L, Chen H, Sun M, Wei Z, Pan C, et al. Early detection of anthracycline-induced cardiotoxicity using two-dimensional speckle tracking echocardiography. Cardiol J. 2013;20(6):592-599.
doi pubmed - Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57(22):2263-2270.
doi pubmed - Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911-939.
doi pubmed - Lopez-Sendon J, Alvarez-Ortega C, Zamora Aunon P, Buno Soto A, Lyon AR, Farmakis D, Cardinale D, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41(18):1720-1729.
doi pubmed - Portugal G, Moura Branco L, Galrinho A, Mota Carmo M, Timoteo AT, Feliciano J, Abreu J, et al. Global and regional patterns of longitudinal strain in screening for chemotherapy-induced cardiotoxicity. Rev Port Cardiol. 2017;36(1):9-15.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


