| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 11, Number 5, October 2020, pages 311-318
Low-Density Lipoprotein Cholesterol Targets in Patients With Coronary Heart Disease in Extremadura (Spain): LYNX Registry
Jose Javier Gomez-Barradoa, b, d, Paula Gomez-Tureganoa, Carolina Ortiz-Cortesa, Jorge Vega-Fernandeza, Marta Gomez-Tureganoa, Francisco Javier Garciperez de Vargasc, Luis Enrique Lezcano Gorta, Zineb Kounkaa, Benjamin Roque Rodrigueza, David Chipayo Gonzalesa, Paloma Perez-Espejoa, Ana Isabel Fernandez-Chamorroa, Maria Beltran Morenoa, Maria Jose Romero Castroa, Maria Victoria Mogollon Jimeneza, Gonzalo Marcos Gomeza, Yolanda Porras Ramosa
aSan Pedro de Alcantara University Hospital, Caceres, Extremadura, Spain
bDepartment of Biomedical Sciences, University of Extremadura, Extremadura, Spain
cHospital of Don Benito-Villanueva, Badajoz, Extremadura, Spain
dCorresponding Author: Jose Javier Gomez-Barrado, San Pedro de Alcantara University Hospital, Ronda de San Francisco s/n. 10002 Caceres, Extremadura, Spain
Manuscript submitted April 23, 2020, accepted May 11, 2020, published online August 1, 2020
Short title: LDL-C Targets in Patients With CHD
doi: https://doi.org/10.14740/cr1079
| Abstract | ▴Top |
Background: Low-density lipoprotein cholesterol (LDL-C) contributes decisively to the development of cardiovascular disease (CVD). In the LYNX registry we determined the rate of achievement of the target value of LDL-C, the use of lipid-lowering therapy (LLT) and the predictive factors of not reaching the target in patients with stable coronary heart disease (CHD).
Methods: LYNX included consecutive patients with stable CHD treated at the University Hospital of Caceres, Extremadura (Spain) from September 2016 to September 2018, and those who must have an LDL-C target below 70 mg/dL according to the European Society of Cardiology (ESC) 2016 guidelines. The variables independently associated with the breach of the LDL-C objective were evaluated by multivariable logistic regression.
Results: A total of 674 patients with stable CHD were included. The average LDL-C levels were 68.3 ± 24.5 mg/dL, with 56.7% showing a level below 70 mg/dL. LLT was used by 96.7% of patients, 71.7% were treated with high-powered statins and 30.1% with ezetimibe. The risk of not reaching the target value of LDL-C was higher in women, in active smokers, and in those who had multivessel CHD or had atrial fibrillation. Patients with diabetes mellitus, those who took potent statins or co-administration treatment with ezetimibe were more likely to reach the target level of LDL-C.
Conclusions: The treatment of dyslipidemia in patients with chronic CHD remains suboptimal; however, an increasing number of very high-risk patients achieve the LDL-C objective, although there is still enormous potential to improve cardiovascular outcome through the use of more intensive LLT.
Keywords: Coronary heart disease; Dyslipidemia; Statin; LDL-C; Spain
| Introduction | ▴Top |
Atherosclerotic cardiovascular disease (CVD) is the leading cause of death in Europe, responsible for 45% of all deaths [1], and this despite the fact that the implementation of healthy lifestyles and pharmacological interventions have contributed to the reduction of approximately one-third of mortality [2, 3].
Chronic coronary heart disease (CHD) continues to be the most prevalent cardiology pathology attended in cardiology consultations and accounts for 50% of patients with heart disease [4].
Low-density lipoprotein cholesterol (LDL-C) is one of the main risk factors for CVD because of its role in the development of atherosclerosis. Scientific evidence has shown that LDL-C is the fundamental cause of CVD and that its intensive reduction with lipid-lowering therapy (LLT) should be part of the treatment of all patients with established CVD [5].
The effectiveness of statins has been demonstrated in numerous studies, not only to reduce LDL-C levels [6], but also in the reduction of cardiovascular events [7], and their effectiveness is favored by a more intensive treatment [8]. In addition, the reduction of LDL-C with other LLT different from statins has also proved the reduction of cardiovascular events. Thus, co-administration therapy with ezetimibe demonstrated in the IMPROVE-IT study the benefit of a non-statin associated with standard LLT with statins to reduce cardiovascular events in patients with CVD [9]. More recently, non-statin LLT that is very effective in reducing LDL-C has been approved such as proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) evolocumab and alirocumab [10, 11].
International clinical practice guidelines set out precise objectives for the treatment of dyslipidemia [12, 13]. Although the classification of cardiovascular risk differs slightly between guidelines, all those that were in force at the time the study was conducted defined a target of LDL-C < 70 mg/dL for patients with a very high risk. However, studies have revealed a significant gap between target LDL-C levels and LDL-C levels measured in different populations, particularly in very high-risk patients. The recent DYSIS II study in 18 countries showed that only 29.4% of patients with stable CHD were on targets [14]. In a recent Spanish multicenter registry carried out in cardiology consultations, which included patients with CHD, only 26% of patients met the LDL-C objective, and this despite the fact that 95.3% of them received LLT [15]. More recently, the LIPICERES registry showed that 52.3% of patients with CHD achieved the LDL-C objective [16]. In the EUROASPIRE V registry, carried out in 29 European countries, only 29% of patients with previous infarction achieved the objective; however, Spain is one of the countries in which this degree of achievement of objectives higher, 48.7% [17].
During the elaboration of this article, the new guidelines of dyslipidemias of the European Society of Cardiology (ESC) have been published [18], where it is established a more demanding LDL-C objective in very high-risk patients, which is reduced to < 55 mg/dL.
The objective of this study was to analyze the frequency and predictive factors of achieving LDL-C objectives in patients with stable chronic CHD reviewed in two cardiology consultations in the province of Caceres (Extremadura, Spain).
| Patients and Methods | ▴Top |
Study design
Observational and cross-sectional study in which all patients with chronic CHD who were reviewed in two cardiology consultations of the Department of Cardiology of the San Pedro de Alcantara de Caceres University Hospital (Extremadura, Spain) from September 2016 to September 2018 were consecutively included. This study was approved by the Clinical Research Ethics Committee of the hospital.
Variables studied
The clinical data were obtained from the clinical history of the patients at the initial and only visit. Data on biodemographic parameters (current age and gender) and anthropometric (weight, height, body mass index (BMI) and abdominal perimeter) characteristics, cardiovascular risk factors (arterial hypertension, diabetes mellitus, dyslipidemia, smoking and family history of premature CHD), vascular disease (cerebral or peripheral), presence of atrial fibrillation, valvulopathies, current clinical and analytical parameters (blood glucose, HbA1c, creatinine, cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), LDL-C and lipoprotein (a) (Lp(a)) were collected likewise, and data related to CHD (age of onset, coronary angiography, number of affected vessels and number of events) were also collected.
The name and daily dose of LLT (statins and/or ezetimibe and/or PCSK9i) were collected. High-intensity statins were considered to be those whose expected reduction in LDL-C was 50% (rosuvastatin 20 mg, atorvastatin 40 and 80 mg per day), according to the ESC 2016 guidelines [12].
Variable definition
A patient was considered to have a stable chronic CHD when he or she fulfilled one or more of the following: coronary stenosis > 50%, assessed by coronary angiography or coronary computed tomography (CT); previous percutaneous coronary intervention (PCI); previous coronary artery bypass graft (CABG); or history of acute coronary syndrome (> 3 months before inclusion).
A patient was classified as diabetic if the diagnosis was recorded in his medical history, if he received antidiabetic medication or if he had a fasting blood glucose > 126 mg/dL or an HbA1c > 6.5%. If it was between 100 and 125 mg/dL or HbA1c between 5.7% and 6.4% without a previous diagnosis of diabetes, it was considered prediabetes.
It was considered as a smoker for those who had consumed at least one cigarette in the last month.
One patient was overweight if he had a body mass index (BMI) between 25 and 30 kg/m2, and was obese if he had a BMI > 30 kg/m2. Abdominal obesity was defined by an abdominal perimeter > 102 cm in men and > 88 cm in women.
LDL-C levels were calculated with Friedewald’s formula
The main variable of the study was the “adequate lipid control”. Following the criteria of the European guideline [12], the concentration of LDL-C < 70 mg/dL was defined as adequate control, thus establishing two groups: LDL-C < 70 mg/dL and LDL-C ≥ 70 mg/dL . The update of the ESC 2019 guidelines for dyslipidemia was published during the preparation of this manuscript, and reduced the concentration of LDL-C to obtain adequate control at < 55 mg/dL [18].
Statistical analysis
In the descriptive analysis, the quantitative variables are shown with measures of central tendency and dispersion (mean and standard deviation), except Lp(a), which does not follow a normal distribution (median and interquartile range), and the qualitative variables such as absolute frequency (n) and relative frequency (%). For the comparisons of means, the Student’s t-test was used and to compare more than two means, the analysis of variance (ANOVA) test was applied. For the Lp(a) analysis, non-parametric tests were used (Mann-Whitney U or Kruskal-Wallis).
Factors associated with non-compliance with LDL-C target values were first analyzed using the Student’s t-test or the Mann-Whiney U-test for continuous variables, and with the Chi-square or Fisher’s exact test for categorical variables. Subsequently, a univariate and multivariable logistic regression analysis was used. The variables included in the multivariate model were those with a value of P < 0.2 in the univariate analysis or variables of clinical interest.
Statistical analysis was performed with the SPSS 21.0 program (SPSS, Inc., IL, USA)
The research meets the guidelines for human studies, and the study protocol has been approved by the hospital’s human research committee. The study was conducted in accordance with the ethical standards of the institution responsible for human beings, as well as with the Declaration of Helsinki.
| Results | ▴Top |
Patient characteristics
A total of 674 consecutive patients were included in the study. The mean age was 65.6 ± 12.1 years and 19.3% were women. The mean age of onset of CHD was 59.3 ± 12.0 years, 25.4% (171) of the patients were older than 75 years and 4% (27) were younger than 45 years; and 29.2% (192) of patients had performed a cardiac rehabilitation program.
Table 1 shows the general characteristics of the study population.
 Click to view | Table 1. General Characteristics of the Study Population (N = 674) |
Coronary angiography was performed in 97.6% of the patients, 44.5% showing coronary lesions of a vessel, 15.6% with coronary revascularization surgery and percutaneous revascularization by 80% angioplasty had been performed, stenting in 35.8% of them, and more than one stent to the rest. One hundred twenty-five (18.5%) patients had presented more than one event.
Lipid concentrations and LLT
The lipid values of the whole sample are shown in Table 2. Plasma Lp(a) levels greater than 50 mg/dL were present in 31.2% of patients.
 Click to view | Table 2. Analytical Values of the Whole Sample (Mean ± Standard Deviation) |
Regarding the consumption of LLT, 96.7% (652) of the patients were treated with statins, without differences between sexes (women 96.9% vs. men 96.7%; P: not significant). Figure 1 shows the distribution of its use. The most used is atorvastatin (68.4%). Of the non-statin lipid-lowering agents, 30.1% received ezetimibe and 1% PCSK9i.
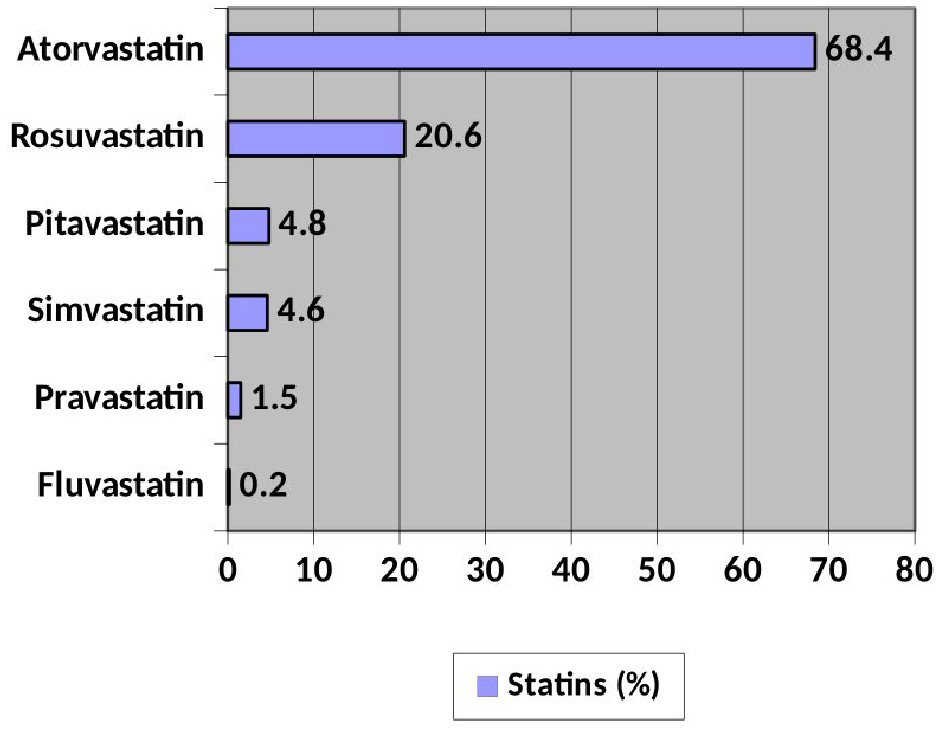 Click for large image | Figure 1. Distribution of use of the different statins. |
Four hundred eighty (71.7%) of the patients were treated with high-intensity statins. There were no significant differences between sexes in the prescription of high-intensity LLT (women 70.0% vs. men 72.1%; P: not significant) nor in that of ezetimibe (women 26.9% vs. men 31.0%; P: not significant).
Predictors of achievement of LDL-C objectives
Three hundred eighty-two patients had a LDL-C < 70 mg/dL (56.7%), and 448 patients with a non-HDL cholesterol < 100 mg/dL (66.5%). One hundred eighty-nine patients had a LDL-C < 55 mg/dL (28%); 59% of men vs. 46.9% of women had LDL-C values < 70 mg/dL (P = 0.014). There were no differences in achieving LDL-C objectives according to the age of the patients.
Neither hypertensive patients, nor diabetics, nor those with overweight or obesity, nor those with a family history of early CHD showed differences in the achievement of LDL-C objectives, but those who had a history of dyslipidemia (49.9% vs. 70.2%; P < 0.0001) did.
Patients who achieved the LDL-C target < 70 mg/dL also showed lower mean HDL-C levels (46.3 ± 14.1 mg/dL vs. 48.5 ± 13.6 mg/dL; P = 0.039) and similar triglyceride values (116.5 ± 75.6 mg/dL vs. 122.4 ± 57.7 mg/dL; P = 0.272). Lp(a) levels were higher in individuals who did not achieve the LDL-C target < 70 mg/dL compared to those who did (42.9 ± 44.4 mg/dL vs. 36.4 ± 39.2 mg/dL; P = 0.035).
In patients who were in high-intensity LLT, the LDL-C target of < 70 mg/dL was achieved in 75.9%, compared to 24.1% of those who did not (P = 0.009); and in those who took ezetimibe, regardless of whether they took high-intensity LLT, the LDL-C target of < 70 mg/dL was achieved at 65.4%, compared to 34.6% of those who did not take it (P = 0.007).
Factors associated with the fulfillment of the LDL-C objective
Table 3 details the characteristics associated with achieving the LDL-C target < 70 mg/dL, among which are diabetes mellitus, a lipid-lowering treatment with high-intensity statins and taking ezetimibe. In contrast, female sex, active smoking, having more extensive coronary involvement, and the coexistence of atrial fibrillation were associated with poor control with LDL-C ≥ 70 mg/dL.
 Click to view | Table 3. Factors Independently Associated With LDL-C < 70 mg/dL (Logistic Regression) |
| Discussion | ▴Top |
In Western countries, the incidence of myocardial infarction has decreased in recent years and long-term survival has improved [19, 20]. However, patients with a previous myocardial infarction continue to have a high cardiovascular risk and it is estimated that one in five has a recurrent cardiovascular event in the following 10 years [21].
The existence of a relationship between the reduction of LDL-C and cardiovascular risk has been shown in numerous studies, such that the reduction of 39 mg/dL (1 mmol/L) is associated with a 23% decrease in cardiovascular events [9]. Therefore, current guidelines recommend, regardless of LDL-C level, the prescription of LTT to all patients with CHD if not contraindicated. In LYNX, 96.7% of all patients were on LLT. Other studies have also reported high percentages of very high-risk patients on LLT, as in the DYSIS II study, in which 93.8% of the CHD cohort used LLT [14].
However, despite the high percentage of very high-risk patients in LLT, most studies have poor results in achieving LDL-C objectives. In the DYSIS II study, only 29.4% of patients with stable CHD achieved the LDL-C target < 70 mg/dL [14]. In patients with the Swedish Secondary Prevention after Heart Intensive Care Admission register, with 1 year of post-infarction follow-up during 2013, about 70% did not reach the LDL-C target < 70 mg/dL (69% of men and 75% of women) [22].
In the CEPHEUS registry, conducted in 29 countries in the Middle East, only 22.8% of patients with CAD achieved the LDL-C target of < 70 mg/dL [23], and in the EPHESUS study, conducted in 40 centers in Turkey, only 18% [24].
In Spain, in the REPAR registry it was achieved a control level of 26% in LDL-C objectives in patients with stable CHD, with great variability in the average levels of LDL-C in different regions [15]. The most recent data in Spain show improvements in the management of dyslipidemia, but the achievement of the LDL-C objective remains insufficient. In the LIPICERES registry, the achievement of LDL-C objectives in patients with stable CHD was 52.3% [16].
In LYNX, more than half of patients with CHD have an LDL-C level below 70 mg/dL, and this is probably related to an increasingly widespread use of LLT and the high-intensity of such treatment.
Data obtained from the Swedish quality registry SWEDEHEART showed an improvement in the achievement of LDL-C control objectives, which was 56% in 2017 and rising to 60% in 2018 [25]. The causes could be greater therapeutic compliance by having greater access to high-intensity statins by lowering the prices of atorvastatin after the patent expired. In previous observational studies, adherence to statin therapy in patients with CHD ranged from 50% to 79%; factors that may affect compliance would include demographic and socioeconomic factors, side effects, lifestyle, distance between doctor visits, and number of pills prescribed [22].
Cross-sectional EUROASPIRE records carried out in several European countries on patients with CHD over the last few years (1999 - 2013) showed an improvement in the achievement of target LDL-C levels, as well as a higher prescription of LLT and a higher use of high-intensity statins over time; the degree of lipid control of LDL-C < 70 mg/dL increased from 6.1% in EUROASPIRE II (1999 - 2000) to 25.6% in EUROASPIRE IV (2012 - 2013), that is only one-fifth of the patients achieved LDL-C treatment objectives [26]. In the recently published EUROASPIRE V, carried out in 2016 - 2017, there has been a slight improvement in the control of LDL-C in secondary prevention [27]; and the global data for Europe show an achievement of 29% objectives, however, the LLT was frankly improvable, since up to 16% of the patients did not take any LLT, and the percentage of combined LLT was quite low (10%). However, the data of the Spanish arm for achieving LDL-C objectives were 49%, and they are very close to the results of LYNX.
Danchin et al have demonstrated similar results in 18 countries in non-Western Europe, in which only 32% of very high-risk patients achieved an LDL-C < 70 mg/dL [28].
The new lipid guides of the ESC 2019 [18] published during the writing of this article recommend LDL-C levels < 55 mg/dL in very high-risk patients; in LYNX, 28% of patients manage to reach that goal.
In LYNX the control of LDL-C seems to be better in men than in women and in diabetic patients compared to those who are not, while smoking was systematically associated with unfavorable lipid levels. LDL-C was better controlled in patients with high-intensity LLT vs. those with low/moderate LLT and in those with combination LLT with ezetimibe. In contrast, control of LDL-C was worse in patients with a big extent of coronary involvement, and in those with atrial fibrillation.
In LYNX, being a woman was associated with a lower achievement of the LDL-C objective < 70 mg/dL (23% vs. 32% in men), and this despite the fact that they received LLT in the same proportion and there were no differences in the prescription of high-intensity LLT or ezetimibe. This difference in LLT according to sex has been previously reported in different studies [14, 29, 30], despite the fact that there are no specific recommendations for the treatment of hyperlipidemia [31] depending on the sex of the patient, due to various factors, such as a lower prescription (not found in LYNX), lower adherence, higher prevalence of intolerance, and higher rate of discontinuation of statin therapy in women, due to lower awareness of cardiovascular risk by the doctor and the patient [32]. In the EUROASPIRE V registry the lipid profile was on average less optimal in women compared to men, with higher levels of CT, LDL-C and non-HDL-C; and showed a LDL-C > 70 mg/dL in 68.6% of men compared to 77.9% of women [27]; but in this registry women received less treatment with LLT and received less frequently LLT of high intensity than men, and this was reflected in their lipid profile, facts that do not occur in LYNX.
The EPHESUS registry [24] included 1,482 secondary prevention patients (63 ± 10 years, 38% women), and only 18% achieved an LDL-C < 70 mg/dL; and as it was seen in other countries, fewer women were treated properly (86% vs. 15%; P = 0.017).
The more powerful statins are known to reduce morbidity and mortality after acute coronary syndrome more effectively than the less powerful ones [33-35]. The most recent European Atherosclerosis Society (EAS)/ESC guidelines of 2016 and 2019 for the treatment of dyslipidemia [12, 18] recommend that LLT with high doses of powerful statins begin early afterwards in all patients with acute coronary syndrome without contraindications or intolerance, regardless of the baseline value of LDL-C. In LYNX, 97.6% of all patients took LLT, of which 71.7% were high-intensity LLT.
The IMPROVE-IT study showed that, in patients with CHD, there was a greater reduction in LDL-C levels with ezetimibe in combination with simvastatin than with simvastatin alone [9]. Current recommendations recommend that if LDL-C target cannot be achieved with high-intensity statins at the maximum tolerated dose, ezetimibe, and if necessary, a PCSK9i should be added [18]. In LYNX, 30.1% of patients received LLT in combination of a statin with ezetimibe. We can improve the degree of lipid control through the progressive titration of statin treatment and the use of combined LLT in a high proportion of patients with CVD.
PCSK9i were used only in seven patients in LYNX, which is probably due to the fact that in the period 2016 - 2018 the use of these drugs had not yet been extended and their availability was limited. The efficacy of PCSK9i has been widely demonstrated [10, 11], but their high cost and the limitations of the Health Administrations continue to be a barrier to their use in the clinic.
In LYNX, treatment with high-intensity statins and with ezetimibe is associated with a lower risk of not reaching the LDL-C objective in the multivariate analysis, in line with previous findings [14, 33]. In Spain, in the REPAR study, the adequate control of LDL-C in secondary prevention was only 26%. Taking into account that only 45% of treated patients took high-intensity statins and that ezetimibe was only used in 14% of patients, the scope for improvement was wide. In addition, this study showed that therapeutic inertia is common in Spain in secondary prevention, since treatment was not extended in 70% of cases [15]. There are several reasons that could explain, at least in part, this insufficient control of LDL-C, and there are included, among others, underestimation of cardiovascular risk, fear of side effects of treatment, poor use of combination therapy lipid lowering or lack of adherence to treatment [15].
Of the EUROASPIRE V patients, 88% were on LTT, although with variations between countries of 75 to 98%; and 59% of them were on high-intensity LLT (72% with atorvastatin 40 - 80 mg/day and in 20% with rosuvastatin 20 - 40 mg/day), while 8% were on combination statin treatment with ezetimibe. Furthermore, in this registry, therapeutic inertia is analyzed 1 year after acute coronary syndrome, verifying that there is an increase in the number of patients without LTT (from 12% at the time of hospital discharge to 16% 1 year later) and the proportion of high-intensity LTT decreased from 58% to 50% 1 year later [27].
Being diabetic is associated with a good compliance of the objectives of LDL-C in repeated studies [36]; also in LYNX. In the article written by Breuker et al [37], performed exclusively in the diabetic population, it is shown a high rate of patients who did not achieve the LDL-C objective in that population, with 59% of patients with LDL-C ≥ 70 mg/dL, and women were also less prone to achieve the goal of LDL-C.
In the Swedish registry there were a smaller proportion of patients with diabetes and patients treated with statins in the uncontrolled group than in the controlled group [22].
The worse control of LDL-C in patients with more extensive coronary disease that shows the LYNX record does not surprise us, since a worse control with higher LDL-C values favor the progression of atherosclerosis. Regarding the relation between poor control of LDL-C and the presence of atrial fibrillation, the evidence supports the role of inflammation in the production of atrial fibrillation, and statins have anti-inflammatory effects that could be relevant for the prevention of fibrillation handset. In a group selected for underlying inflammation of individuals in the JUPITER trial, increasing levels of C-reactive protein were associated with an increased risk of atrial fibrillation and random association to rosuvastatin significantly reduced that risk [38]. Therefore, patients with more potent LLT and, therefore, lower LDL-C, would have lower inflammation and less incidence of atrial fibrillation. In a recent study, the use of statins tended to be associated with a lower risk of new onset atrial fibrillation after a heart attack, and statins tended to reduce new onset atrial fibrillation after heart attack [39].
In our work, the mean levels of Lp(a) are higher in patients with LDL-C > 70 mg/dL. We know that the determination of LDL-C includes the cholesterol content of Lp(a), which can contribute up to 30-45% of LDL-C levels [40]. This circumstance makes it more difficult for individuals with elevated Lp(a) levels to lower LDL-C levels below 70 mg/dL [41].
Conclusions
The results of the LYNX study show that despite the evidence in reducing the risk of CVD through intensive secondary prevention, the management of LDL-C in these patients remains suboptimal, and there is still a large treatment gap between the guidelines and the achievement of the LDL-C objective. However, an increasing number of very high-risk patients achieve the LDL-C objective, although there is still enormous potential to improve cardiovascular outcome through the use of more intensive LLT and combined therapies.
Limitations of the study
Some limitations must be recognized. First, the monocentric nature of our study could limit the generalization of our result. Second, our center is especially motivated and involved in cardiovascular risk control, and this may have contributed to the LDL-C control results obtained.
Acknowledgments
None to declare.
Financial Disclosure
This research has not received any funding.
Conflict of Interest
There are no conflicts of interest.
Informed Consent
Written informed consents were obtained from all patients.
Author Contributions
All authors participated in data acquisition. JJ Gomez-Barrado conducted the original study; JJ Gomez-Barrado and P Gomez-Turegano performed the secondary data analysis and the development of the original manuscript. JJ Gomez-Barrado, P Gomez-Turegano and C Ortiz contributed to writing of the manuscript, as well as critically analyzing the content and improving the quality of the manuscript. All authors discussed the results and contributed to the final versions of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe 2015: epidemiological update. Eur Heart J. 2015;36(40):2673-2674.
doi - Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120(2):366-380.
doi pubmed - Hartley A, Marshall DC, Salciccioli JD, Sikkel MB, Maruthappu M, Shalhoub J. Trends in mortality from ischemic heart disease and cerebrovascular disease in Europe: 1980 to 2009. Circulation. 2016;133(20):1916-1926.
doi pubmed - Cordero A, Bertomeu-Martinez V, Mazon P, Facila L, Cosin J, Bertomeu-Gonzalez V, Rodriguez M, et al. Patients with cardiac disease: Changes observed through last decade in out-patient clinics. World J Cardiol. 2013;5(8):288-294.
doi pubmed - Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472.
doi pubmed - Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297.
doi pubmed - Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532-2561.
doi - Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Raber L, Mach F, Windecker S. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J. 2018;39(14):1172-1180.
doi pubmed - Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387-2397.
doi pubmed - Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722.
doi pubmed - Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107.
doi pubmed - Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999-3058.
doi pubmed - Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1-87.
doi pubmed - Gitt AK, Lautsch D, Ferrieres J, De Ferrari GM, Vyas A, Baxter CA, Bash LD, et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: Results from the Dyslipidemia International Study II. Atherosclerosis. 2017;266:158-166.
doi pubmed - Galve E, Cordero A, Cequier A, Ruiz E, Gonzalez-Juanatey JR. Degree of Lipid Control in Patients With Coronary Heart Disease and Measures Adopted by Physicians. REPAR Study. Rev Esp Cardiol (Engl Ed). 2016;69(10):931-938.
doi - Gomez-Barrado JJ, Ortiz C, Gomez-Turegano M, Gomez-Turegano P, Garciperez-de-Vargas FJ, Sanchez-Calderon P. [Lipid control in patients with coronary artery disease in a healthcare area in Caceres (Spain): LIPICERES study]. Clin Investig Arterioscler. 2017;29(1):13-19.
doi pubmed - Kotseva K, De Backer G, De Bacquer D, Ryden L, Hoes A, Grobbee D, Maggioni A, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol. 2019;26(8):824-835.
doi pubmed - Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188.
doi pubmed - Nichols M, Townsend N, Scarborough P, Rayner M. Trends in age-specific coronary heart disease mortality in the European Union over three decades: 1980-2009. Eur Heart J. 2013;34(39):3017-3027.
doi pubmed - Gomez-Barrado JJ, Ortiz-Cortes C, Gomez-Turegano P, Lezcano-Gort LE, Kounka Z, Romero-Castro MJ. Cambios en el sindrome coronario agudo en una decada en un hospital de referencia provincial. Clin Investig Arterioscler. 2019;31:93-100.
doi pubmed - Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36(19):1163-1170.
doi pubmed - Journath G, Hambraeus K, Hagstrom E, Pettersson B, Lothgren M. Predicted impact of lipid lowering therapy on cardiovascular and economic outcomes of Swedish atherosclerotic cardiovascular disease guideline. BMC Cardiovasc Disord. 2017;17(1):224.
doi pubmed - Chiang CE, Ferrieres J, Gotcheva NN, Raal FJ, Shehab A, Sung J, Henriksson KM, et al. Suboptimal Control of Lipid Levels: Results from 29 Countries Participating in the Centralized Pan-Regional Surveys on the Undertreatment of Hypercholesterolaemia (CEPHEUS). J Atheroscler Thromb. 2016;23(5):567-587.
doi pubmed - Mert GO, Basaran O, Mert KU, Dogan V, Ozlek B, Celik O, Ozlek E, et al. The reasons of poor lipid target attainment for secondary prevention in real life practice: Results from EPHESUS. Int J Clin Pract. 2019;73(9):1-9.
doi pubmed - SWEDEHEART Annual report 2018. http://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter. Accessed on January 2, 2020.
- Kotseva K, Wood D, De Bacquer D, De Backer G, Ryden L, Jennings C, Gyberg V, et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol. 2016;23(6):636-648.
doi pubmed - De Backer G, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Z, Ryden L, Tokgozoglu L, et al. Management of dyslipidaemia in patients with coronary heart disease: Results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis. 2019;285:135-146.
doi pubmed - Danchin N, Almahmeed W, Al-Rasadi K, Azuri J, Berrah A, Cuneo CA, Karpov Y, et al. Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: The International ChoLesterol management Practice Study (ICLPS). Eur J Prev Cardiol. 2018;25(10):1087-1094.
doi pubmed - De Smedt D, De Bacquer D, De Sutter J, Dallongeville J, Gevaert S, De Backer G, Bruthans J, et al. The gender gap in risk factor control: Effects of age and education on the control of cardiovascular risk factors in male and female coronary patients. The EUROASPIRE IV study by the European Society of Cardiology. Int J Cardiol. 2016;209:284-290.
doi pubmed - Gulati M, Merz CN. Advances in lipid therapy: the role of lipid treatment in women in primary prevention. Prog Cardiovasc Dis. 2016;59(2):178-189.
doi pubmed - Goldstein KM, Zullig LL, Bastian LA, Bosworth HB. Statin Adherence: Does Gender Matter? Curr Atheroscler Rep. 2016;18(11):63.
doi pubmed - Schoen MW, Tabak RG, Salas J, Scherrer JF, Buckhold FR. Comparison of adherence to guideline-based cholesterol treatment goals in men versus women. Am J Cardiol. 2016;117(1):48-53.
doi pubmed - Murphy SA, Cannon CP, Wiviott SD, de Lemos JA, Blazing MA, McCabe CH, Califf RM, et al. Effect of intensive lipid-lowering therapy on mortality after acute coronary syndrome (a patient-level analysis of the Aggrastat to Zocor and Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 trials). Am J Cardiol. 2007;100(7):1047-1051.
doi pubmed - Ribeiro RA, Ziegelmann PK, Duncan BB, Stella SF, da Costa Vieira JL, Restelatto LM, Moriguchi EH, et al. Impact of statin dose on major cardiovascular events: a mixed treatment comparison meta-analysis involving more than 175,000 patients. Int J Cardiol. 2013;166(2):431-439.
doi pubmed - Josan K, Majumdar SR, McAlister FA. The efficacy and safety of intensive statin therapy: a meta-analysis of randomized trials. CMAJ. 2008;178(5):576-584.
doi pubmed - Stone MA, Charpentier G, Doggen K, Kuss O, Lindblad U, Kellner C, Nolan J, et al. Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care. 2013;36(9):2628-2638.
doi pubmed - Breuker C, Clement F, Mura T, Macioce V, Castet-Nicolas A, Audurier Y, Boegner C, et al. Non-achievement of LDL-cholesterol targets in patients with diabetes at very-high cardiovascular risk receiving statin treatment: Incidence and risk factors. Int J Cardiol. 2018;268:195-199.
doi pubmed - Pena JM, MacFadyen J, Glynn RJ, Ridker PM. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33(4):531-537.
doi pubmed - Tseng CH, Chung WJ, Li CY, Tsai TH, Lee CH, Hsueh SK, Wu CC, et al. Statins reduce new-onset atrial fibrillation after acute myocardial infarction: A nationwide study. Medicine (Baltimore). 2020;99(2):e18517.
doi pubmed - Yeang C, Witztum JL, Tsimikas S. 'LDL-C' = LDL-C + Lp(a)-C: implications of achieved ultra-low LDL-C levels in the proprotein convertase subtilisin/kexin type 9 era of potent LDL-C lowering. Curr Opin Lipidol. 2015;26(3):169-178.
doi pubmed - Tsimikas S, Stroes ESG. The dedicated "Lp(a) clinic": A concept whose time has arrived? Atherosclerosis. 2020;300:1-9.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


