| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 2, April 2022, pages 73-80
The Association of N-Terminal Pro-Brain Natriuretic Peptide With Time to Clinical Worsening in Hispanic Patients With Pulmonary Arterial Hypertension
Yacoub Khataba, Sayed Reshad Ghafourib, Haider Alkhateebc, Debabrata Mukherjeec, Hernando Garciad, Nils Patrick Nickele, f
aFoster School of Medicine, Texas Tech University Health Sciences Center, El Paso, TX 79905, USA
bDepartment of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX 79905, USA
cDivision of Cardiology, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX 79905, USA
dDivision of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL 33140, USA
eDivision of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX 79905, USA
fCorresponding Author: Nils Patrick Nickel, Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX 79905, USA
Manuscript submitted February 3, 2022, accepted March 24, 2022, published online April 5, 2022
Short title: NT-proBNP and TTCW in Hispanic PAH Patients
doi: https://doi.org/10.14740/cr1362
| Abstract | ▴Top |
Background: Measurement of N-terminal pro-brain natriuretic peptide (NT-proBNP) level is an important parameter in the risk assessment of patients with pulmonary arterial hypertension (PAH). Data about the prognostic value of NT-proBNP in the Hispanic PAH population are lacking. Historically, clinical trials in PAH have only included a minority of Hispanic patients. It has been reported that baseline NT-proBNP levels differ between different ethnicities. Furthermore, NT-proBNP levels can be impacted by declining renal function, making its interpretation difficult regarding clinical decision making.
Methods: In a retrospective single-center cohort analysis, Hispanic patients with PAH had a baseline outpatient NT-proBNP level drawn during a period of clinical stability and were followed for 1 year to monitor for time to clinical worsening (TTCW). The association of baseline NT-proBNP and TTCW was assessed in patients with normal and abnormal renal function.
Results: A total of 26 patients (22%) met the clinical endpoint of clinical worsening. Twenty-seven patients (24%) had chronic kidney disease (CKD). At baseline NT-proBNP levels showed a significant inverse correlation with 6-min walk test (6MWD, r = -0.382, P = 0.02), and a significant positive correlation with renal function (r = 0.273, P = 0.05). NT-proBNP levels did not correlate with age (r = 0.19, P = 0.11) or body mass index (BMI) (r = -0.292, P = 0.061). NT-proBNP levels of > 1,415 ng/L were significantly associated with shorter TTCW (P < 0.01) in all patients and in patients with CKD (P = 0.03). A stepwise increase in NT-proBNP levels by 100 ng/L was associated with a higher risk of meeting the clinical endpoint of TTCW in patients with normal renal function (hazard ratio (HR) = 1.8, P < 0.01) and CKD (HR = 1.5, P < 0.01).
Conclusions: In Hispanic patients with PAH, NT-proBNP is a valuable tool to predict 1-year TTCW, independent of renal function.
Keywords: Pulmonary arterial hypertension; N-terminal pro-brain natriuretic peptide; Hispanics
| Introduction | ▴Top |
Pulmonary arterial hypertension (PAH) is a progressive disease of the distal pulmonary arteries that leads to progressive pressure overload of the right heart and eventually right heart failure if left untreated [1]. Volume or pressure overload of the right ventricle is associated with the natriuretic peptide system activation. The pro-brain natriuretic peptide (proBNP) is secreted by cardiomyocytes and cleaved into an inactive N-terminal proBNP (NT-proBNP) fragment and active hormone BNP [2]. Given the prolonged half-life of NT-proBNP and a more accurate assay system, it is preferably used in clinical practice. In PAH, BNP, and NT-proBNP levels correlate with right-sided hemodynamics, time to clinical worsening (TTCW), and overall survival in PAH [3]. Reduction in NT-proBNP levels after the initiation of PAH-targeted therapy is associated with favorable outcomes [4]. However, NT-proBNP levels can be influenced by non-cardiac factors, such as renal function, inflammation, body weight, age, gender, diurnal cycle and ethnicity [5-7]. Therefore, it is difficult to determine a single disease-specific cutoff value for prognostication in PAH. In the current guidelines, NT-proBNP levels < 300 ng/L are desired in patients with PAH, a value that is mainly based on expert opinion [8]. However, these thresholds have proven excellent prognostic value [3].
There is cumulating evidence that health disparities have direct impact on clinical outcomes of minorities in the USA. This also applies to Hispanic patients with PAH [9, 10]. Unfortunately, patient registries lack sufficient representation of ethnic minorities. It has been shown that Hispanic patients with PAH seem to have more severe PAH at the time of presentation, compared to other ethnicities [11-13]. Despite differences in disease severity, previous studies have also reported racial differences in baseline NT-proBNP levels. In the Diabetes Prevention Program, Hispanic patients had significantly lower NT-proBNP baseline levels compared to White or American-Indian study subjects [14]. Whereas the Dallas Heart study found lower baseline NT-proBNP levels in African-Americans, but no difference between White and Hispanic subjects [7]. Certainly, a significant research gap exists in the clinical characteristics in racial subgroups within the PAH population.
The prognostic value of NT-proBNP in Hispanic patients with PAH has not been established, making it difficult for physicians to risk-assess Hispanic PAH patients. This study examines the clinical usefulness of NT-proBNP as a biomarker to predict clinical worsening in a Hispanic PAH cohort with and without chronic kidney disease (CKD).
| Patients and Methods | ▴Top |
Patients
This single-center cohort consisted of 115 Hispanic patients with group-1 World Health Organization (WHO) PAH followed at the Pulmonary Hypertension Clinic at the Texas Tech Health Science Center in El Paso, TX between March 2017 and October 2021. Other WHO- pulmonary hypertension groups and non-Hispanic patients were excluded from this study.
Each patient’s chart was studied retrospectively by two investigators, the diagnosis and outcomes were validated by the treating PAH physician. Comorbidities, renal function, functional capacity, and demographics were collected from the time of the initial outpatient clinic visit used for this study during a phase of clinical stability. Patients were followed for 1 year after the initial visit and TTCW was recorded. Race and ethnicity were self-disclosed by the patients. In all patients, the diagnosis of PAH was based on hemodynamic definitions and clinical classification according to the Sixth World Symposium on Pulmonary Hypertension in 2018 [15]. Hemodynamics were measured by right heart catheterization and non-WHO-group 1 Hispanic patients forms were excluded by various laboratory studies, echocardiography, pulmonary function testing, chest X-ray, ventilation-perfusion scanning, and chest computed tomography angiography, and/or pulmonary angiography, if necessary. Right heart hemodynamics and 6-min walk test (6MWD) were obtained within 3 - 6 months of the baseline NT-proBNP level. CKD was defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 for 3 months or more. Glomerular filtration rate (GFR) was estimated with the Modification of Diet in Renal Disease (MDRD) study equation [16]. Only patients with stable renal function (stable GFR for at least 3 months) were included, patients with end-stage renal disease or worsening renal function were excluded. The study was approved by the Texas Tech University Institutional Review Board in accordance with the national legislation and the institutional requirements (IRB #E22054 and IRB #E21091).
Follow-up and outcome parameters
TTCW was defined as the time of the first event of clinical worsening after NT-proBNP levels were obtained in the outpatient setting. Clinically relevant events were retrospectively identified from patient charts and were defined as: 1) decrease of more than 15% in 6MWD; 2) escalation of PAH-targeted therapy due to signs and symptoms of progressive right heart failure; 3) hospitalization for right heart failure; 4) referral for lung transplant; 5) death related to right heart failure. Follow-up was concluded on October 1, 2021.
Statistical analyses
Data are presented as absolute numbers, percentages, mean (standard deviation), or median (interquartile range). Differences of means between baseline variables were assessed by Student’s t-test for parametric, Mann-Whitney U test for non-parametric data, and Chi-square test was used to compare ordinal data. Receiver operating characteristic (ROC) analyses were used to define the cutoff value of NT-proBNP to predict clinical worsening (highest sum of sensitivity and specificity). Kaplan-Meier plots were used to illustrate the timing of events (TTCW) during follow-up in relation to NT-proBNP levels after 1 year; and statistical assessment was performed by the log-rank test. The effect of increments of NT-proBNP levels by 100 ng/L was assessed using univariate Cox regression analysis. For all analyses, P values less than 0.05 were considered statistically significant. All calculations were performed using SPSS 27 software.
| Results | ▴Top |
Patients’ characteristics and outcomes
A total of 115 Hispanic patients (70%) were identified out of 164 prevalent group-1 WHO PAH patients. In this cohort, 109 patients (67%) were females (Table 1), and 21 patients (18%) received triple combination PAH-targeted therapies (Table 2). Out of the 115 patients enrolled in this analysis, 26 patients (23%) met the primary endpoint of clinical worsening over 1-year period; 27 patients (24%) were diagnosed with CKD, based on the definition mentioned in the methodology section (Table 3). Patients with clinical worsening had lower WHO functional class, lower 6MWD, higher mean right atrial pressure, and higher NT-proBNP levels at baseline (Table 1). In patients with CKD, mean serum creatinine (SCr) was 1.3 mg/dL, compared to 0.7 mg/dL in patients without a CKD diagnosis. Patients with CKD had lower WHO functional class and higher NT-proBNP levels at baseline (Table 3, Fig. 1). Stratification by REVEAL Lite 2.0 showed that 41 patients (36%) were in the low, 33 (29%) in the intermediate, and 41 (36%) in the high-risk categories. REVEAL Lite 2.0 was significantly associated with TTCW after 1 year (unadjusted hazard ratio (HR): 1.6, 95% confidence interval (CI): 1.3 - 1.9, P < 0.05). An elevated SCr at baseline was also associated with 1-year TTCW (unadjusted HR:1.8, 95% CI: 1.1 - 3.0, P < 0.05).
 Click to view | Table 1. Baseline Characteristics in Patients With and Without Clinical Worsening |
 Click to view | Table 2. Pulmonary Arterial Hypertension Targeted Therapies at Baseline Visit |
 Click to view | Table 3. Baseline Characteristics in Patients With and Without CKD |
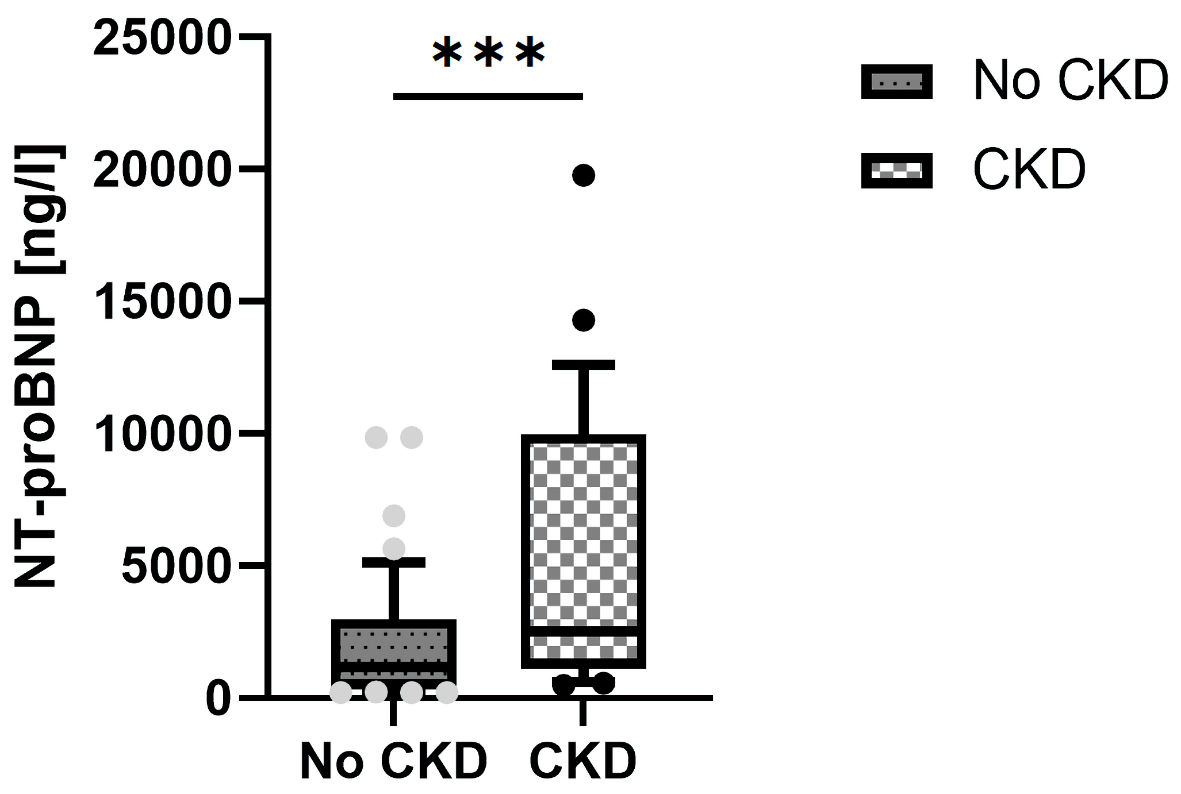 Click for large image | Figure 1. NT-proBNP levels in patients with and without CKD. NT-proBNP: N-terminal pro-brain natriuretic peptide; CKD: chronic kidney disease. |
Prediction of clinical worsening by NT-proBNP levels
During 1-year follow-up after the baseline NT-proBNP level was obtained, clinical worsening occurred in 26 out of 115 patients (23%) at a mean time of 243 days. Causes for clinical worsening were admission for worsening PAH (n = 12, 45%), decrease of more than 15% in 6MWD (n = 5, 21%), escalation of PAH-targeted therapy (n = 7, 25%), and death (n = 2, 9%).
ROC analysis revealed that NT-proBNP was a strong predictor of clinical worsening (area under the ROC curve (AUC): 0.89, 95% CI: 0.8 - 0.9). The European Society of Cardiology guidelines recommended NT-proBNP level above 300 ng/L predicted TTCW over 1 year with a specificity of 100% and sensitivity of 51% (Fig. 2). The best NT-proBNP cutoff level for predicted TTCW over 1 year was 1,415 ng/L with a sensitivity of 64% and specificity of 85% (Fig. 3). An unadjusted Cox proportional hazard analysis confirmed a significant association of NT-proBNP and TTCW after 1 year (HR: 1.7, 95% CI: 1.4 - 2.3, P < 0.05). NT-proBNP levels were independently associated with TTCW when adjusted for age, gender, and renal function (HR: 1.6, 95% CI: 1.3 - 2.1, P < 0.05).
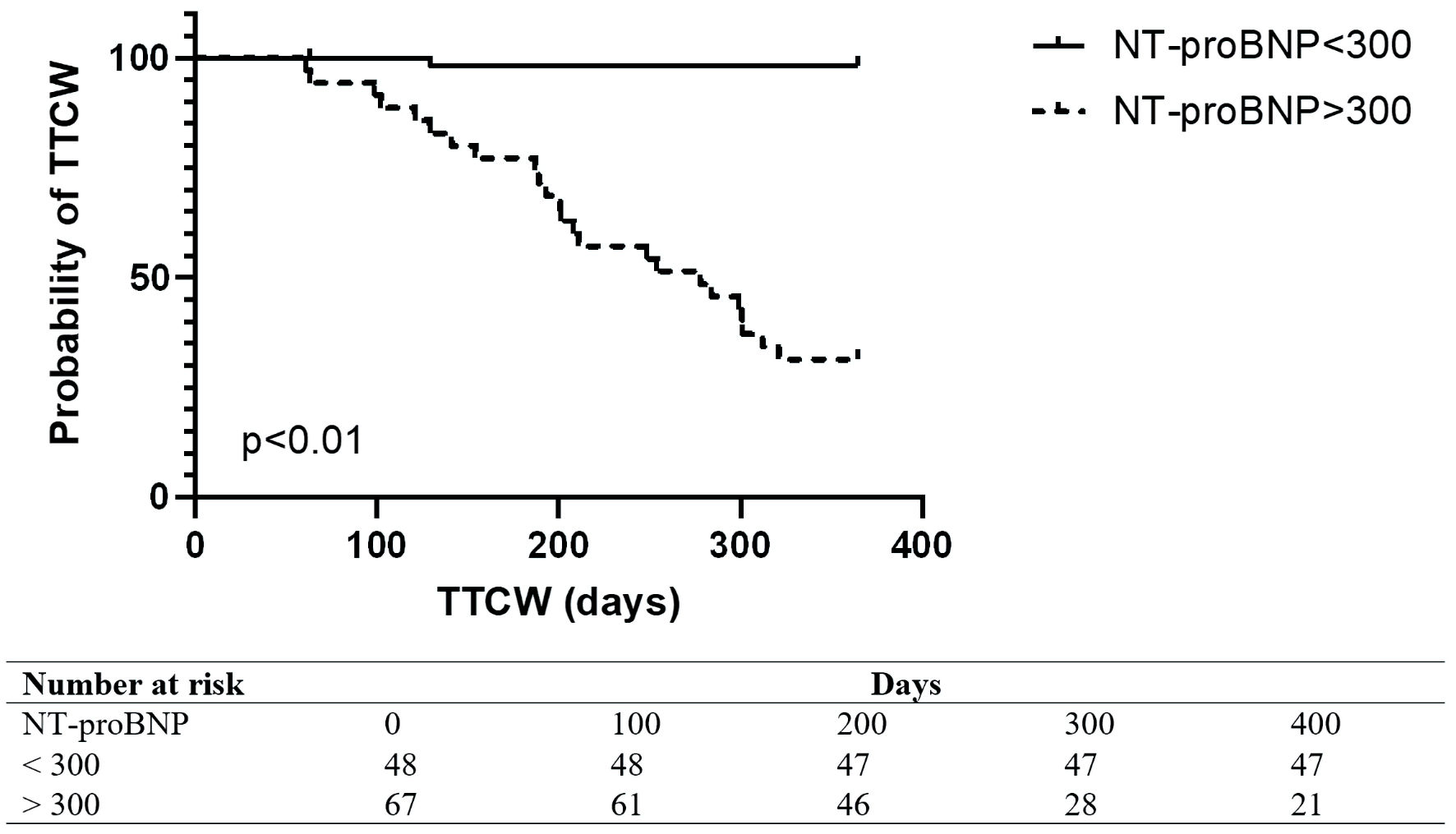 Click for large image | Figure 2. Kaplan Meier Plot in all patients with NT-proBNP levels below or above 300 ng/L. TTCW: time to clinical worsening; NT-proBNP: N-terminal pro-brain natriuretic peptide. |
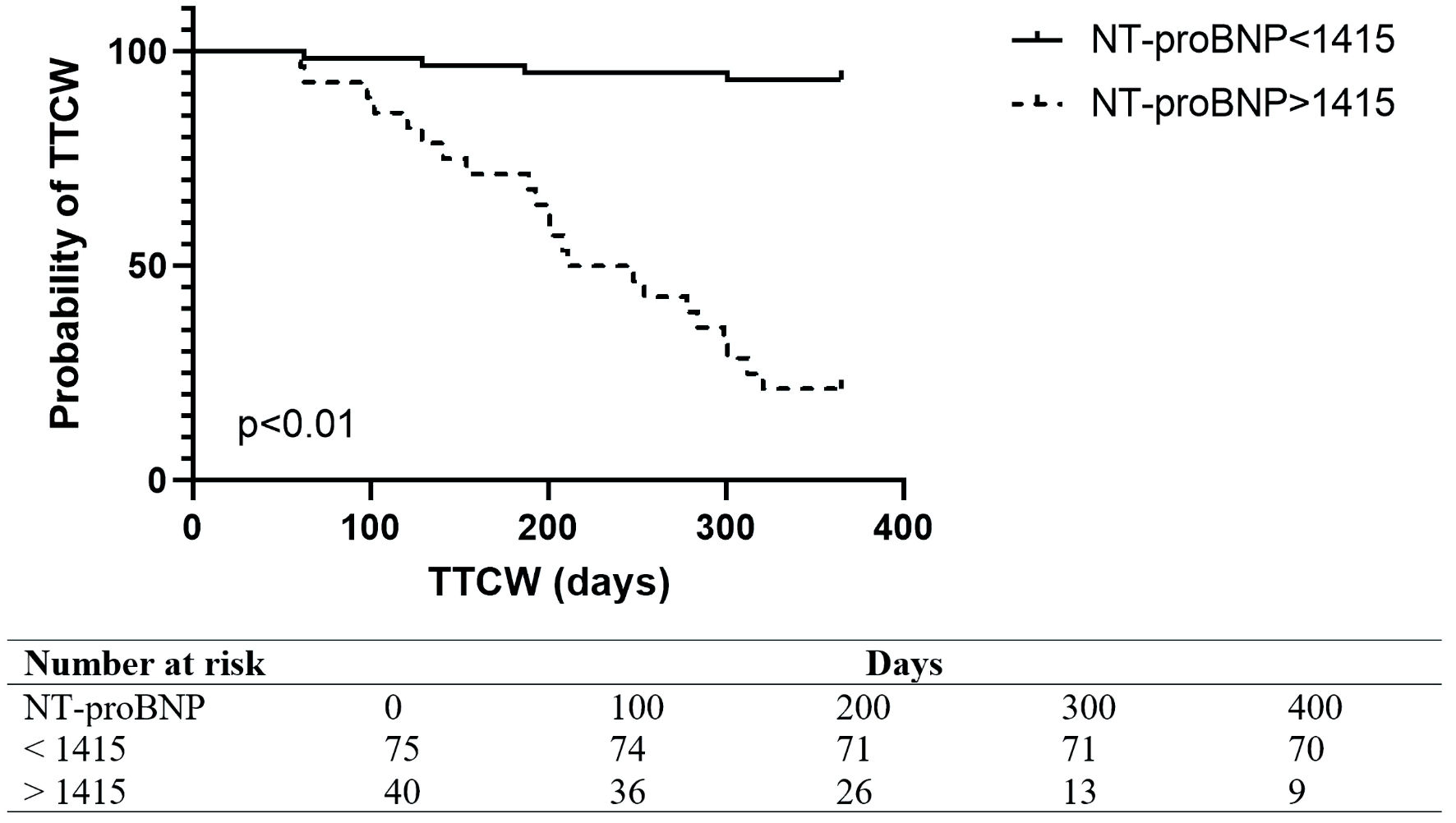 Click for large image | Figure 3. Kaplan Meier Plot in all patients with NT-proBNP levels below or above 1,415 ng/L. TTCW: time to clinical worsening; NT-proBNP: N-terminal pro-brain natriuretic peptide. |
Prediction of clinical worsening by NT-proBNP levels in the setting of CKD
Out of 115 patients, 27 (23%) patients had baseline CKD as defined above. Out of those 27 patients, 11 (41%) had a clinical worsening event over the 1-year period. Clinical worsening occurred at a mean time of 205 days (± 160 days). NT-proBNP levels were higher in patients with CKD, compared to patients with normal renal function (P < 0.05) (Fig. 1). ROC analysis showed that NT-proBNP had a high sensitivity and specificity in predicting 1-year TTCW (AUC: 0.89) (Fig. 4). A ROC-derived NT-proBNP cutoff of 1,415 ng/L significantly predicted TTCW after 1 year (HR log-rank P = 0.05) (Fig. 5). Cox regression analysis showed that increasing NT-proBNP levels were significantly associated with TTCW in patients with CKD (HR: 1.5, 95% CI: 1.1 - 2.1, P < 0.05).
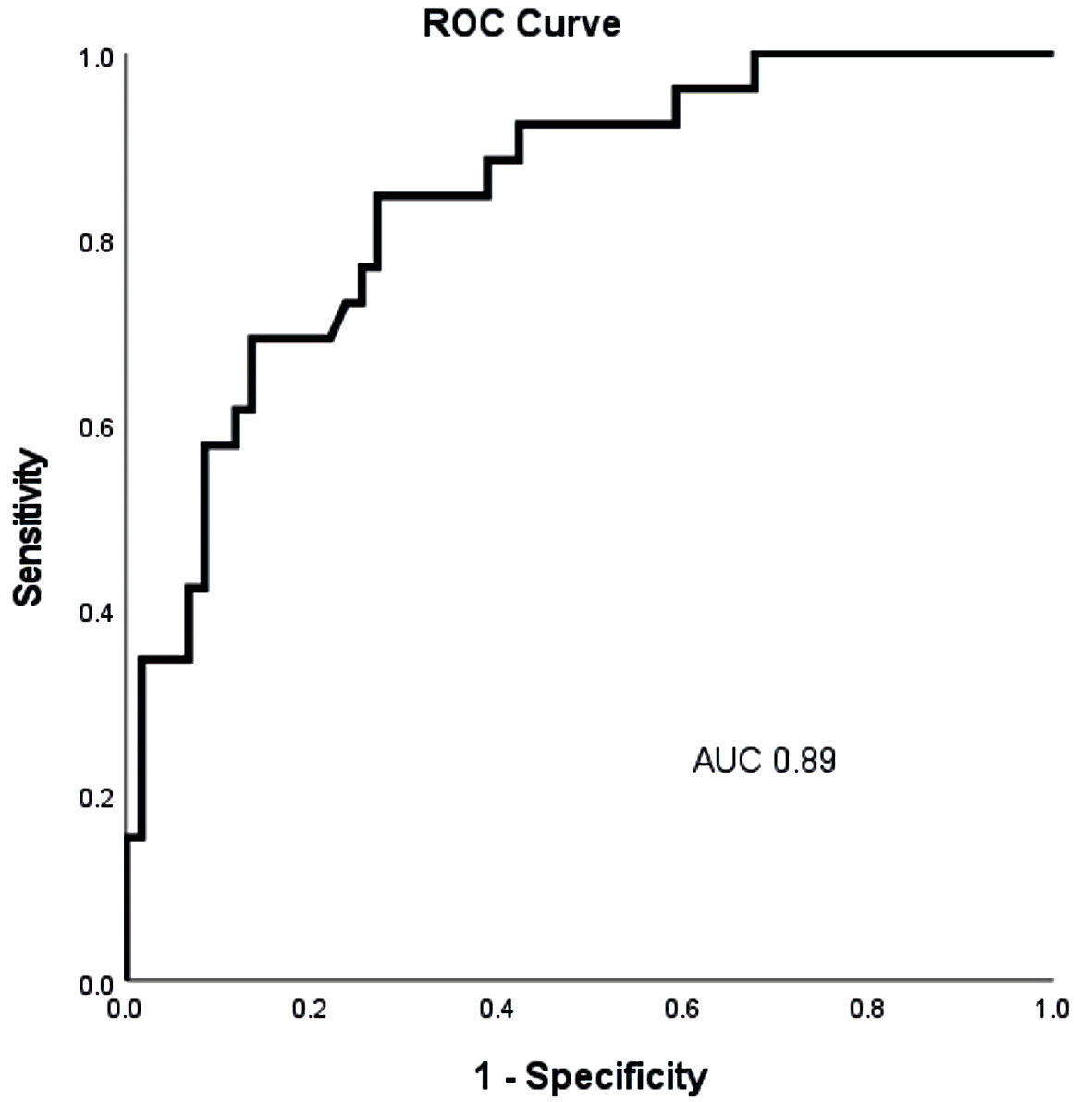 Click for large image | Figure 4. Receiver operating characteristic curve analysis of baseline NT-proBNP levels to predict 1-year time to clinical worsening (TTCW) in all patients. NT-proBNP: N-terminal pro-brain natriuretic peptide; AUC: area under the curve; TTCW: time to clinical worsening. |
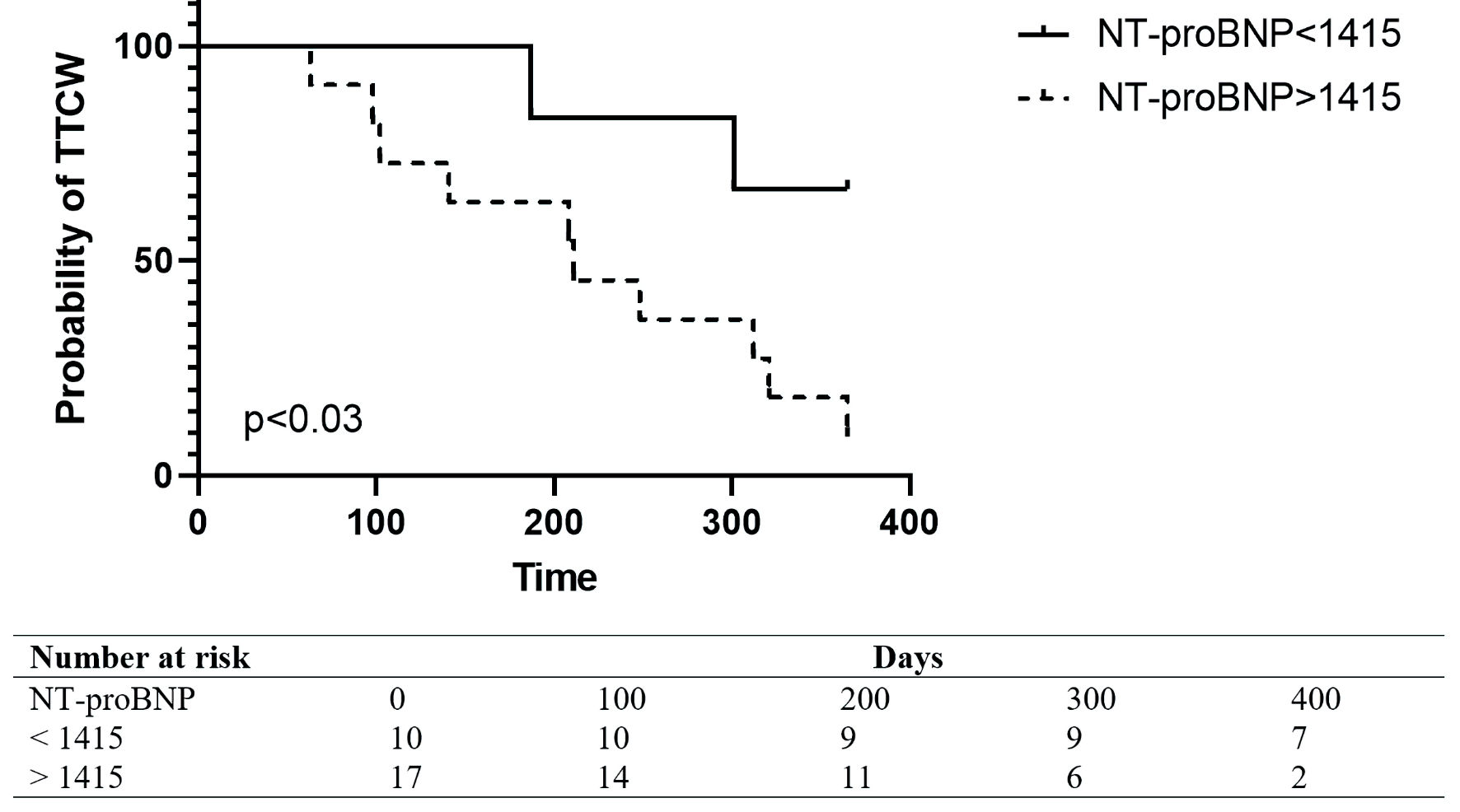 Click for large image | Figure 5. Kaplan Meier Plot in patients with CKD and BNP levels below or above 1,415 ng/L. TTCW: time to clinical worsening; NT-proBNP: N-terminal pro-brain natriuretic peptide. |
| Discussion | ▴Top |
To the best of the author’s knowledge, this is the first analysis of the predictive value of NT-proBNP levels in a Hispanic-only PAH cohort. Our data confirm that NT-proBNP is a powerful marker of risk stratification, and its value also applies in Hispanic patients with PAH independent of renal function. In this cohort, elevated NT-proBNP predicted 1-year TTCW with a sensitivity of 64% and specificity of 85%. The predictive value of TTCW of NT-proBNP remained significant in patients with CKD.
NT-proBNP levels are influenced by various parameters such as age, gender, body mass index (BMI), renal function, and ethnicity. It has been reported that Hispanic individuals may have a relative lower baseline NT-proBNP level when compared to Caucasians. Most national and international PAH-registries lack robust data on Hispanic individuals. The REVEAL registry for instance only enrolled 9% of Hispanic PAH patients [17]. Most clinical trials and registries did not include “Hispanic” as a separate ethnicity [18]. The clinical phenotype, risk profile, and the response to PAH-targeted therapy in Hispanic patients are largely unknown. However, there is evidence that Hispanic patients present with PAH-features associated with worse survival when compared to Caucasians [11, 12]. These findings could not be reproduced in a more recent analysis of the REVEAL cohort, where Hispanic patients had similar survival when compared to other ethnic groups [13]. Traditional predictors of poor outcome in PAH have not been vigorously studied in this patient population and clinicians have difficulties to extrapolate established risk predictors to this patient population. Here, we show that NT-proBNP levels carry a strong predictive value for 1-year TTCW in a solely Hispanic PAH population. Our study confirms that NT-proBNP is a useful biomarker in predicting 1-year TTCW in Hispanic patients. The cutoff values for NT-proBNP for optimal prognostication in PAH are unknown and likely depend on the study population and the underlying PAH etiology. PAH guidelines suggest that NT-proBNP levels below 300 ng/L are associated with low risk for clinical worsening [19]. In our cohort, patients with NT-proBNP of 300 ng/L or below had an excellent 1-year prognosis. On the contrary, an NT-proBNP level above 1,400 ng/L was suggested to identify patients with high risk for clinical worsening [19]. The ROC-derived cut-off in our cohort is very close to this value. Taken together, our data suggest that established NT-proBNP values can be used to risk-stratify Hispanic PAH patients. This is in line with a recent multi-ethnic analysis that did not find significant differences in baseline NT-proBNP levels between Hispanic and non-Hispanic PAH patients [13].
In our cohort, 24% of the study population had CKD. That is in line with other studies, reporting a CKD prevalence between 4% and 46%, depending on definition of CKD and the PAH patient population studied [20]. CKD is an important comorbidity in PAH and is associated with poor survival. Furthermore, impaired renal function could impair the interpretation of NT-proBNP levels, since this peptide is at least partially cleared by the kidneys [2, 21]. However, several reports have established the predictive value of NT-proBNP in patients with impaired renal function [22]. One report studied the prognostic value of NT-proBNP in a pre-specified PAH population with CKD [23]. The authors found that NT-proBNP remained a strong predictor of outcome in patients with CKD, independent of the severity of renal impairment. The ROC-derived cut-off of 1,415 ng/L performed well in our Hispanic PAH CKD cohort and was significantly associated with TTCW, making it a useful biomarker in this patient population.
Limitations
There are several limitations to this study. The small sample size, retrospective design, single-cohort setting, varying observational periods and different medical management strategies limit the interpretation of our study results. Lack of follow-up data do not allow to establish if NT-proBNP values correlate with a favorable response to PAH-targeted therapy. Furthermore, the study is limited by the lack of hemodynamic data in relation to NT-proBNP levels. Thus, it does not allow any conclusions to whether NT-proBNP levels correlate with established markers of PAH in Hispanic patients. The single-center cohort in a US-Mexio border city do not allow generalizability to the global Hispanic community. Patients with end-stage renal disease or with worsening renal function were excluded from this study, making the interpretation of NT-proBNP in these patients difficult.
Conclusions
NT-proBNP is a valid biomarker to predict TTCW in Hispanic patients with PAH. NT-proBNP correlated with renal function in this population but remains a valid biomarker for clinical worsening even in patients with stable CKD. Despite this, more research is needed to allow improved risk stratification in Hispanic patients with PAH.
Acknowledgments
The authors have no acknowledgement to declare.
Financial Disclosure
The authors received no funding or grant for their work in preparation of this manuscript.
Conflict of Interest
There is no potential conflict of interest to be declared by the authors.
Informed Consent
Written informed consent was not required for this study in accordance with Texas Tech University Institutional Review Board.
Author Contributions
NPN conceptualize the study, obtained the IRB approval, performed the data analysis, and prepared the first draft of the article. YK and SRG helped with data collection, design the table and figures, and edit the manuscript. HA, DM, and HG reviewed the article, did critical corrections and final approval. All authors read and agreed to the submission of final version of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Galie N, Channick RN, Frantz RP, Grunig E, Jing ZC, Moiseeva O, Preston IR, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889.
doi pubmed - Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL, Jr. Biology of the natriuretic peptides. Am J Cardiol. 2008;101(3A):3-8.
doi pubmed - Chin KM, Rubin LJ, Channick R, Di Scala L, Gaine S, Galie N, Ghofrani HA, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139(21):2440-2450.
doi pubmed - Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, Westerkamp V, Welte T, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;39(3):589-596.
doi pubmed - Battistoni A, Rubattu S, Volpe M. Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. Int J Cardiol. 2012;157(2):160-168.
doi pubmed - Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, McGill C, et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92(5):628-631.
doi - Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial differences in natriuretic peptide levels: the Dallas heart study. JACC Heart Fail. 2015;3(7):513-519.
doi pubmed - Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. [2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension]. Kardiol Pol. 2015;73(12):1127-1206.
doi pubmed - Talwar A, Garcia JGN, Tsai H, Moreno M, Lahm T, Zamanian RT, Machado R, et al. Health disparities in patients with pulmonary arterial hypertension: a blueprint for action. An Official American Thoracic Society Statement. Am J Respir Crit Care Med. 2017;196(8):e32-e47.
doi pubmed - Al-Naamani N, Paulus JK, Roberts KE, Pauciulo MW, Lutz K, Nichols WC, Kawut SM. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ. 2017;7(4):793-796.
doi pubmed - Usmani A, Vergis T, Ostrower A, Machado RF. Minority patients with pulmonary arterial hypertension present with more severe disease at the time of diagnosis. American Thoracic Society Thematic Poster Session. B72. Others Here with us: Clinical Features of Pulmonary Vascular Disease I. 2017;195:A4232.
- DeJesus Perez V, Badesch D, Zamanian RT, Fineman J, Klinger JR, Ford J, McConnell JW. Characterization of hispanics with pulmonary hypertension in the us: the pulmonary hypertension association registry. American Thoracic Society Thematic Poster Session. B72. Others Here with us: Clinical Features of Pulmonary Vascular Disease I. 2017;195:A4255.
- Medrek S, Sahay S, Zhao C, Selej M, Frost A. Impact of race on survival in pulmonary arterial hypertension: Results from the REVEAL registry. J Heart Lung Transplant. 2020;39(4):321-330.
doi pubmed - Gupta DK, Walford GA, Ma Y, Jarolim P, Wang TJ, DPP Research Group. Racial/ethnic differences in circulating natriuretic peptide levels: The Diabetes Prevention Program. PLoS One. 2020;15(2):e0229280.
doi pubmed - Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):801913.
doi pubmed - Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089-2100.
doi pubmed - Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164-172.
doi pubmed - Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galie N, Ghofrani HA, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522-2533.
doi pubmed - Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903-975.
doi pubmed - Nickel NP, O'Leary JM, Brittain EL, Fessel JP, Zamanian RT, West JD, Austin ED. Kidney dysfunction in patients with pulmonary arterial hypertension. Pulm Circ. 2017;7(1):38-54.
doi pubmed - Tsutamoto T, Sakai H, Yamamoto T, Nakagawa Y. Renal clearance of N-terminal pro-brain natriuretic peptide is markedly decreased in chronic kidney disease. Circ Rep. 2019;1(8):326-332.
doi pubmed - Horii M, Matsumoto T, Uemura S, Sugawara Y, Takitsume A, Ueda T, Nakagawa H, et al. Prognostic value of B-type natriuretic peptide and its amino-terminal proBNP fragment for cardiovascular events with stratification by renal function. J Cardiol. 2013;61(6):410-416.
doi pubmed - Harbaum L, Hennigs JK, Baumann HJ, Luneburg N, Griesch E, Bokemeyer C, Grunig E, et al. N-terminal pro-brain natriuretic peptide is a useful prognostic marker in patients with pre-capillary pulmonary hypertension and renal insufficiency. PLoS One. 2014;9(4):e94263.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


