| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Review
Volume 9, Number 2, April 2018, pages 75-82
Main Considerations of Cardiogenic Shock and Its Predictors: Systematic Review
Maria Christiane Valeria Braga Braile-Sternieria, Eliana Migliorini Mustafaa, Victor Rodrigues Ribeiro Ferreiraa, b, Sofia Braile Sabinoa, Giovanni Braile Sternieria, Lucia Angelica Buffulin de Fariaa, Bethina Canaroli Sbardellinia, Cibele Olegario Vianna Queiroza, Domingo Marcolino Brailea, Idiberto Jose Zotarelli Filhoa, c
aDomingo Braile Institute of Sao Jose do Rio Preto (SP), Rua Luiz Vaz de Camoes, 3111 - Vila Redentora, Sao Jose do Rio Preto - SP, 15015-750, Sao Paulo, Brazil
bFaceres - Medical School of Sao Jose do Rio Preto, Av. Anisio Haddad, 6751 - Jardim Francisco Fernandes, Sao Jose do Rio Preto - SP, 15090-305, Sao Paulo, Brazil
cCorresponding Author: Idiberto Jose Zotarelli Filho, Domingo Braile Institute of Sao Jose do Rio Preto (SP), Rua Luiz Vaz de Camoes, 3111 - Vila Redentora, Sao Jose do Rio Preto - SP, 15015-750, Sao Paulo, Brazil
Manuscript submitted March 26, 2018, accepted April 10, 2018
Short title: Cardiogenic Shock and Its Predictors
doi: https://doi.org/10.14740/cr715w
- Abstract
- Introduction
- Methods
- Literature Review and Discussion
- Hemodynamic and Metabolic Control
- Conclusion
- References
| Abstract | ▴Top |
The mortality rate of post-infarction cardiogenic shock (CS) was 80.0-90.0%. Recent studies show a significant reduction of hospital mortality to approximately 50.0%. CS is defined as systemic tissue hypoperfusion resulting from systolic and/or diastolic heart dysfunction, the main cause of which is acute myocardial infarction (AMI). The main predictors are biological markers such as troponin, CKMB and lactate. A systematic literature review and meta-analysis is performed in order to present and correlate the main literary findings on CS and its evolution with possible changes in biomarkers such as troponin, lactate and CKMB. After criteria of literary search with the use of the mesh terms: cardiogenic shock; acute myocardial infarction; biomarkers; troponin; CKMB; lactate; clinical trials and use of the bouleanos “and” between the mesh terms and “or” among the historical findings. In the main databases such as Pubmed, Medline, Bireme, EBSCO, Scielo, etc., a total of 96 papers that were submitted to the eligibility analysis were collated and, after that, 41 studies were selected, following the rules of systematic review - PRISMA (Transparent reporting of systematic reviews and meta-analyzes-http://www.prisma-statement.org/). Some risk factors for its development in AMI are advanced age, female gender, anterior wall infarction, diabetes mellitus, systemic arterial hypertension, previous history of infarction and angina. The CS associated with AMI depends on its extent and its complications, being the main ones: mitral regurgitation, rupture of the interventricular septum and rupture of the free wall of the left ventricule. The diagnosis is based on the clinical manifestations, such as mental confusion, oliguria, hypotension, tachycardia, fine pulse, sweating, and cold extremities; in hemodynamic aspects: systolic blood pressure was < 90.0 mm Hg or 30 mm Hg below baseline, pulmonary capillary pressure was > 18.0 mm Hg and cardiac index was < 2.2 L/min/m2. Laboratory and imaging exams should be requested to evaluate the possible etiology of CS, its systemic repercussions and comorbidities. The treatment aims at the rapid reestablishment of the blood flow in the affected artery, to improve the patient’s prognosis. The biomarkers dosage in the daily clinical practice of the different cardiological centers can facilitate the diagnosis and the conduction of the dubious cases and the best evaluation of the degree of myocardial suffering after CS.
Keywords: Cardiogenic shock; Acute myocardial infarction; Biomarkers; Troponin; CKMB; Lactate; Clinical trials
| Introduction | ▴Top |
Cardiogenic shock (CS) is a clinical syndrome characterized by tissue hypoperfusion resulting from cardiac failure associated with high mortality [1, 2]. The heart, in the absence of hypovolemia, is unable to maintain adequate blood flow to meet the tissues’ metabolic needs, with progressive and potentially irreversible organic dysfunction occurring. It is the most common cause of death after acute myocardial infarction (AMI) [3].
Despite the therapeutic advances, there was no significant reduction in its incidence. Historically, the mortality rate of post-infarction CS was 80.0-90.0% [2]. Recent studies show a significant reduction of hospital mortality to approximately 50.0%. It is believed that this improvement in prognosis may be related to early reperfusion therapies, promoting reduction in the size of the ischemic area [1]. The recognition of reversible factors and the aggressive and early treatment of the acute phase of the condition are of great importance for improving the prognosis [2].
Several causes may compromise cardiac performance with consequent reduction of supply of oxygen to tissues and onset of CS status [3, 4]. Examples of biological parameters such as troponin, lactate, glycemia and CKMB may point to the evolution to CC. The most common etiology of CS is AMI with an estimated incidence of 5.0-8.0% [3-5]. This complication may occur as a consequence of any type of acute coronary syndrome, but more frequently in AMI with ST segment elevation [5].
The extent of myocardial infarction, with involvement greater than 40.0% of left ventricular muscle mass, is associated with the occurrence of CS. Mechanical complications of the infarct are also cause of shock [6]. Rupture of the interventricular septum, rupture of the free wall of the left ventricle and rupture of the papillary muscle with the installation of acute mitral insufficiency [7]. The diagnoses of these mechanical complications are very important since specific therapeutic measures should be instituted immediately [8].
In this context, severe myocardial ischemia, which occurs in cases of AMI or in some patients with unstable angina, results in injury followed by the release of cellular constituents into the bloodstream [8, 9]. Thus, in clinical practice, increases in myocardial isoform creatine kinase (CKMB) and lactic dehydrogenase rates are interpreted as markers of myocardial cell damage. The evaluation of the activity of these enzymes can be done quickly and at low cost and in routine situations are satisfactory parameters to confirm the diagnosis, to monitor the evolution and to estimate the size of the myocardial infarction (MI). There are limitations since specificity is compromised in cases of associated skeletal muscle involvement and, in addition, sensitivity is low in the early hours of evolution due to the delayed appearance of these markers in the blood [10]. It should be added that the sensitivity of CKMB is not high enough to detect small myocardial damage, due to the analytical inaccuracy of the measures of activity and the wide range of normality [11]. This led to the search for other methods or new diagnostic markers of myocardial cell injury, trying to overcome the limitations.
Fatty acid-linked protein (H-FABP), which comprises from 15.0% to 30.0% of all cytoplasmic proteins, resembles myoglobin with respect to variations in serum concentration, having slightly higher specificity [12]. Kleine et al [13] compared plasma levels of H-FABP with those of CKMB and alpha-hydroxybutyrate dehydrogenase (alpha-HBDH) in patients with AMI. The maximum normal values of H-FABP (19 µg/L), CKMB (10 U/L) and alpha-HBDH (160 U/L) obtained in the plasma dosing of 72 blood donors served as the limit value for comparison. H-FABP levels were significantly higher than the normal 3-h limit, with peak H-FABP, CKMB and alpha-HBDH peaks at 4.1 ± 0.9, 8.4 ± 1.4 and 25.0 ± 9.5 h after AMI, respectively, indicating that H-FABP is more adequate than CKMB and alpha-HBDH for the early diagnosis of AMI. Glycogen phosphorylase is also a cytosolic protein with large cardiogenic specificity with kinetics of its BB isoform similar to that of myoglobin.
Troponins have received increasing attention as highly specific markers of cellular injury. Troponins form a complex that regulates the calcium-dependent interaction of myosin and actin [14]. They are constituted of three different proteins (troponin I, C and T) existing in both skeletal and cardiac muscle and encoded by different genes. Troponin C is identical in both skeletal and cardiac muscle but the coding genes for troponin I and cardiac and skeletal T are different, which allowed extremely low cross-reactivity monoclonal antibodies to be developed to facilitate the diagnosis of AMI. In patients with AMI, elevated creatine phosphokinase activity above normal values is rarely found 4 to 6 h after pain onset, making early diagnosis strongly dependent on typical electrocardiographic changes. This becomes a problem because the electrocardiogram (ECG) is inconclusive in up to 40.0% of patients [15, 16].
Cardiac troponin I is not expressed in human skeletal muscle during fetal development, following skeletal muscle trauma or during the regeneration of this type of muscle. Unlike CKMB, cardiac troponin I is highly specific for myocardial tissue, is not detectable in the blood of healthy people, shows a proportionally much greater increase above the limit values in cases of MI and can remain elevated for 7 to 10 days after the acute episode [17]. In addition, a troponin I assay was implanted in the evaluation of precordial pain in patients seeking the emergency department of UCLA Medical Center. With the help of this test, it was possible to safely exclude the existence of MI and in less than half the time with the traditional approach using CKMB [18].
Myocardial dysfunction (systolic and diastolic) leads to hypotension, reduction of cardiac output and reduction of coronary perfusion pressure, with progressive loss of myocardial function and increase of left ventricular end-diastolic pressure with consequent pulmonary congestion and hypoxia. This establishes a vicious cycle with progressive decrease of cardiac output, worsening of tissue perfusion and of the heart itself, particularly in the presence of obstructive coronary disease. Within this vicious cycle, there is also the participation of activated neurohormonal mechanisms with an initial goal of maintaining perfusion to vital organs [19]. There is increased activity of the sympathetic nervous system and the renin-angiotensin-aldosterone system, triggering peripheral vasoconstriction and post-load increase in addition to salt and water retention, increasing intravascular volume and preload. In this condition, the activated mechanisms become inappropriate and lead to progressive worsening of ventricular performance [20].
Laboratory tests, such as blood count, renal function, blood glucose, lactate blood gas, coagulogram, CKMB and troponin, and imaging should be requested to evaluate the systemic repercussions of the shock, to identify its probable etiology and comorbidities [21]. The ECG is important to diagnose AMI and arrhythmias as causes of shock. Chest X-ray shows cardiac area and possible pulmonary congestion. The echocardiogram detects areas of hypokinesia, akinesia or dyskinesia, in addition to diagnosing mechanical complications of AMI that cause shock. Coronary angiography allows the diagnosis and treatment of CS due to AMI [22].
New evidence suggests that, in parallel, a systemic inflammatory response occurs, with activation of the complement system, release of inflammatory cytokines, production of nitric oxide and inappropriate peripheral vasodilation, which is another factor involved in the reduction of systemic and coronary perfusion [23]. It is also worth mentioning that in situations of shock, there is prioritization of blood flow to the heart and brain, to the detriment of surgical organs, generating ischemia of the intestinal mucosa. This component facilitates bacterial translocation and the onset of infection. The occurrence of positive cultures may reach more than 70.0% in these patients, and decreased values of systemic vascular resistance should be considered as additional sepsis [23, 24].
The factors associated with a higher chance of progression to CS after AMI are: age, anterior wall infarction, presence of ST elevation, previous history of angina, infarction and/or heart failure, multiarterial coronary disease, and diabetes mellitus. In the studies, GUSTO I10 and GUSTO III [25], the main predictors of CS were: age, heart rate, systolic blood pressure (SBP) and Killip functional class. Regarding time as a factor of occurrence of CS, it is worth mentioning that only 20.0% of post-infarction shocks are present at hospital admission. Therefore, during the hospitalization continuous observation of the patient should be kept, aiming at the early recognition of clinical signs and laboratory indicators of shock [26]. Patients with AMI sometimes present complications such as arrhythmias, pulmonary congestion, systemic arterial hypertension and pain during the initial course. The treatment instituted for these complications may precipitate the installation of shock in patients at risk [27].
The aim of the present study was to perform a systematic review in order to present the main literary findings on CS and its evolution with the possible alterations of the biomarkers such as troponin, lactate and CKMB.
| Methods | ▴Top |
Study design
Following the criteria of literary search with the use of the mesh terms that were cited in the item below on “search strategies”, a total of 96 papers that were submitted to the eligibility analysis were cross-checked and, after that, 41 studies were selected, following the rules of systematic review - PRISMA (Transparent reporting of systematic reviews and meta-analyzes-http://www.prisma-statement.org/), according to Figure 1.
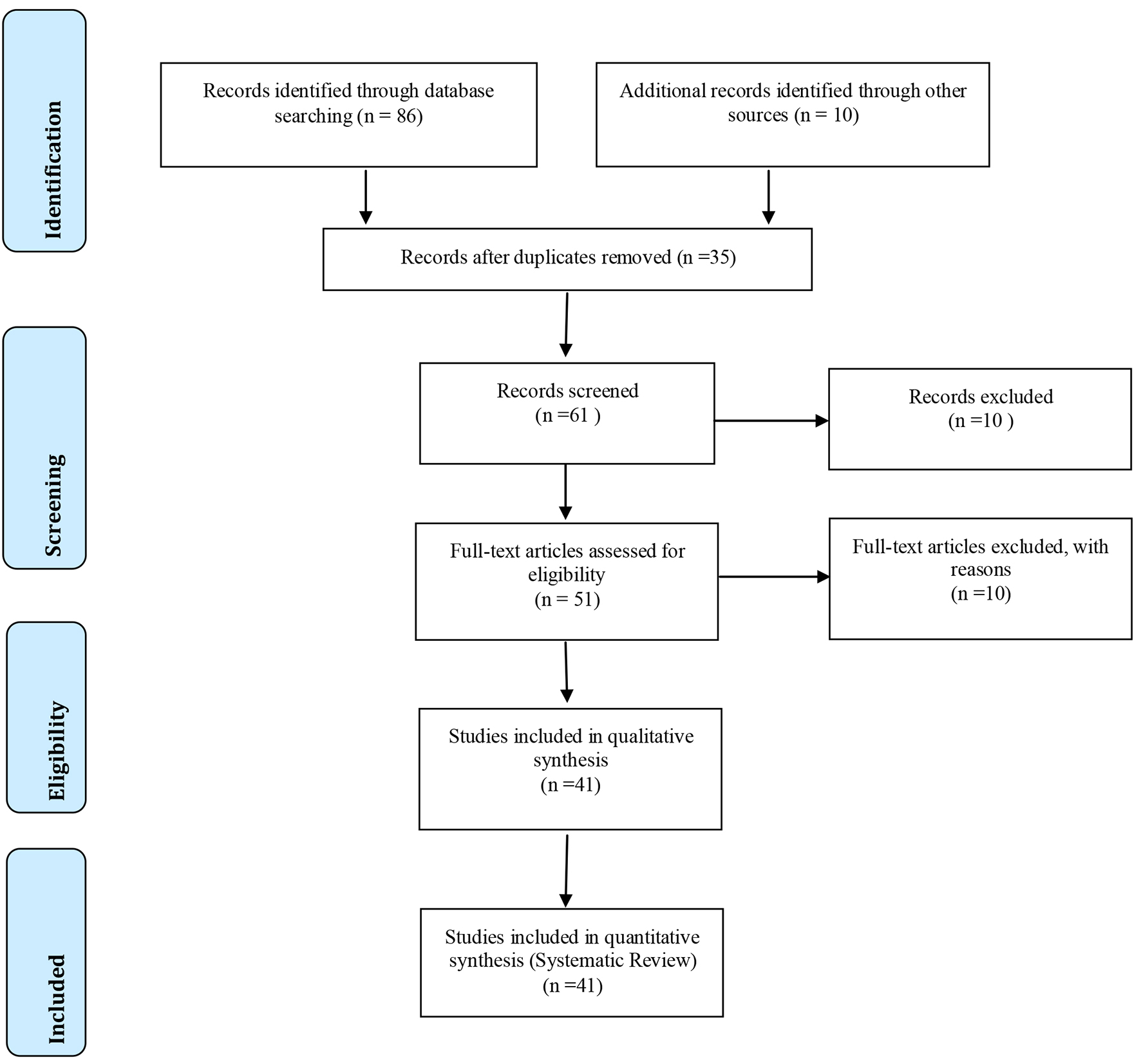 Click for large image | Figure 1. Flowchart. |
Sources of information
The review protocol was based on the criteria of literary search with the use of mesh terms in the main databases such as Pubmed, Medline, Bireme, EBSCO, Scielo, etc. All references are registered in EndNote by the site: http://www.myendnoteweb.com/EndNoteWeb.html?cat=myrefs&.
Search strategy
In general, as an example, the search strategy in MEDLINE/Pubmed, Web of Science, ScienceDirect Journals (Elsevier), Scopus (Elsevier), and OneFile (Gale) followed the following steps: search for mesh terms (cardiogenic shock; acute myocardial infarction, biomarkers, troponin, CKMB, lactate, clinical trials); use of the bouleanos “and” between mesh terms and “or” among the historical findings.
| Literature Review and Discussion | ▴Top |
CS is a clinical situation in which there is progressive deterioration of cardiac function together with poor systemic perfusion and functional organ failure. It is a syndrome that involves the whole circulatory system, with complex neurohormonal mechanisms participating in the genesis of symptoms [28, 29].
Mair et al [30] demonstrated that the first sign of elevation of troponin concentrations in patients with MI occurred with a 3.5 h evolution in 50.0% of the cases, requiring an average of 4.75 h in order to obtain same rate of impairment with CKMB. With 7 h of evolution, 95.0% of the patients presented troponin alteration, fact only matched with CKMB after 12 h onset of symptoms.
Troponin I and troponin T become measurable 3 to 4 h after the initiation of AMI. Studies of peak troponin T levels measured within the first 24 h after admission to selected small groups of patients with precordial pain have demonstrated an excess of cardiac events in patients with troponin T elevation, even in those without CKMB elevation [31-33]. Hamm et al [34] prospectively investigated the usefulness of T and I troponin dosing in the evaluation of patients with acute precordial pain. For that, 773 patients who had precordial pain of less than 12 h but without elevation of the ST segment to the echocardiogram were followed. They found that troponin T was positive in 123 (16.0%) patients and troponin I in 171 (22.0%). Among the 47 patients who progressed to MI, troponin T was positive in 44 (94.0%) and troponin I was positive in all 47 patients. Among the 315 patients with unstable angina, T and I troponin positivity were 22.0% and 36.0%. Both troponins proved to be independent predictors of cardiac events. The rate of events, death or non-fatal infarction, in patients with negative tests was extremely low (1.1 and 0.3 for troponins T and I, respectively).
Luscher et al [35] also sought to determine the applicability of T and I troponins to the risk stratification of patients with unstable coronary heart disease, concluding that both provide independent prognostic information regarding death and MI. The predictive capacity of the markers varied according to the cutoff level, but was already significant with values of 0.05 µg/L for troponin T and 1.5 µg/L for troponin I. They affirm that prospective studies may indicate if troponin T and troponin I will be able to identify patients who will benefit from antithrombotic treatment and/or invasive procedures.
One of the great advantages of troponin dosing rather than CKMB is that it reaches peak values of up to more than 40 times the detection limit, while this is restricted to six to nine times. It is also known that both troponin I and troponin T have equivalent sensitivity for the diagnosis of myocardial cell injury. Wu et al [36] serially measured plasma concentrations of myoglobin, CKMB and troponin I in 25 patients with a confirmed diagnosis of AMI and in 74 patients with suspected infarction but in whom this diagnosis was later ruled out. The cutoff value at troponin I concentration was determined to be 2.5 ng/mL. Of the three markers, myoglobin was the one with the highest sensitivity (50.0%) when blood was collected in the first 6 h after pain onset. All the markers used showed high sensitivity (> 93.0%), 6 to 24 h after the onset of symptoms. The CKMB remained with high sensitivity for 48 h, whereas troponin I was very sensitive up to 72 h. Between 72 and 150 h, the troponin I still showed sensitivity of 70.0% whereas with myoglobin and CKMB the sensitivity was 21.0% and 18.0%, respectively. The specificity of troponin I for patients without MI confirmation was equivalent to that of CKMB and significantly higher than for myoglobin.
In critically ill patients, there may be unrecognized cardiac involvement. Guest and colleagues [31] sought to determine the importance of this occurrence and therefore developed a blind, prospective study in the intensive care units of an academic medical center. A total of 209 patients with a daily troponin I dosage were evaluated. Of these, 32 (15.0%) presented evidence of myocardial damage based on elevated troponin I levels. Only 12 (37.0%) of these 32 patients had been diagnosed of AMI, being no longer clinically recognized in the other 20. Unrecognized impairment was more common in young and blacks. Mortality in patients with recognized myocardial impairment was 42.0% in the non-recognized and higher than in those without compromised (15.0%).
The specificity of troponin I in 59 patients with chronic renal failure, skeletal muscle disease or muscle trauma was greater than that of all other markers including troponin T. Jaffe et al [32] sought the relative sensitivities of troponin I and isoenzymes of lactate dehydrogenase over time in the diagnosis of MI. They found that troponin I was at least as sensitive as lactic dehydrogenase isoenzymes, since 90.0% of patients with AMI still maintained troponin I concentrations above normal even on the fourth day after admission to the coronary unit.
Bertinchant et al [33] found that in patients admitted to the intensive care unit with AMI, troponin I was elevated in all cases and detection was earlier after the onset of pain (4.5 ± 2.3 h) than for CKMB (6.3 ± 3.6 h, P = 0.003). Peak values for troponin I and CKMB occurred 12.2 ± 4.6 h and 15.8 ± 9.0 h, respectively, in patients treated with thrombolysis and the plasma disappearance of troponin I occurred between 5 and 9 days after onset of pain, much later than for CKMB (P = 0.0001). They were also able to document in 49 patients submitted to thrombolysis that the comparative sensitivity of the two tests, admitting normal limits of 0.1 ng/mL for troponin and 15 IU/L for CKMB, based on the first sample harvested - day 3.4 ± 1.3 h after the onset of pain - was 61% and 22%, respectively (P = 0.0002).
Troponin I was not detected in the plasma of 145 normal subjects nor in any of the six patients with severe muscle trauma or rhabdomyolysis, resulting in a specificity of 100.0%. Mair et al [30] demonstrated that the release of troponin I in patients with AMI correlated with infarct size. In a large group of patients admitted for evaluation of suspected AMI, the sensitivity of troponin T (64.0%) was higher than that of other markers, such as CKMB activity and myoglobin level. The specificity, however, was lower than that of CKMB and myoglobin due to 37.0% of false-positive results in patients with unstable angina.
| Hemodynamic and Metabolic Control | ▴Top |
Associated with the anamnesis and the detailed physical examination, the hemodynamic and metabolic parameters are of great use to evaluate the cardiac origin shock. Hemodynamic monitoring should include invasive measurement of systemic blood pressure, central venous pressure measurement, and pulmonary artery catheter measurements [36]. The data obtained from hemodynamic and laboratorial monitoring can be grouped into parameters of macrohemodynamics and global microhemodynamics, the latter reflecting the patient’s perfusional and metabolic status [37].
The main parameters of macrohemodynamics are listed as blood pressure, peripheral perfusion/capillary filling time, diuresis, central venous pressure, cardiac output, and pulmonary artery occlusion pressure. The parameters of microhemodynamics are central venous saturation, arterial lactate, and excess bases [37, 38].
In addition, interventions such as prophylaxis of deep venous thrombosis and gastrointestinal bleeding (especially when the patient is on mechanical ventilation for more than 48 h or with coagulation dysregulation). Initially, one should proceed as the conduct in any shock: to perform volume replacement - as long as there is no clinical and radiological pulmonary congestion - aiming at correcting hypovolemia and hypotension, offering adequate oxygen and ventilation, correcting possible electrolytic and/or metabolic disturbances and arrhythmias potentially compromising cardiac output. The glycemia should be maintained between 150 and 180 mg/dL [38, 39].
The use of inotropes in CS should be initiated in patients with inadequate tissue perfusion and adequate volume. The most commonly used drug is dobutamine, which has a positive inotropic action by the predominant beta-adrenergic effect and the dose to be used can reach up to 20.0 µg/kg/min. In very hypotensive patients, initially a vasopressor agent (dopamine or noradrenaline) was used because the action of peripheral vasodilation of dobutamine may worsen coronary perfusion [40].
In general, inotropes promote hemodynamic improvement in the short term (SBP < 70 mm Hg), the agent to be used is noradrenaline, because its beta-adrenergic action promotes an increase in myocardial contractility, chronotropism and due to the preponderant alpha-agonist effect, there is a significant increase in systemic arterial resistance, with an increase in mean arterial pressure, despite increasing oxygen consumption and increasing cardiac work [41].
A study comparing the efficacy of dopamine and noradrenaline in various types of shock has been published. In the subgroup with CS, there were better outcomes with the use of noradrenaline, and further studies are still needed for this validation [13]. Inhibitors of phosphodiesterase (milrinone) have inotropic effect similar to dobutamine, but with associated vasodilator effect. Therefore, special attention should be given to the risk of worsening of hypotension and increased occurrence of arrhythmias. Some studies have evaluated the effects of levosimendan, a calcium sensitizing drug, on low post-infarct output with favorable results [22].
Vasodilators act in a beneficial way in the pathophysiology of CS by reducing post-load, thus increasing cardiac output [2, 3]. When the pressure is stabilized (SBP > 85 - 90 mm Hg), the use of systemic vasodilators is considered, especially those of arterial and venous action, such as sodium nitroprusside. They are the main therapeutic strategies in patients with optimized blood volume. In cases of patients with acute ischemic syndromes, nitroglycerin use is preferred. In the evidence of pulmonary edema with adequate perfusion, diuretics is associated, always remembering that excessive diuresis can result in severe intravascular depletion, maintaining hypotension, hypoperfusion, infarct extension, and ischemia, and adding dysfunction to the already compromised left ventricle [4].
Among the mechanical circulatory assistance devices, the intra-aortic balloon (IAB) is the most widely used device available in clinical practice. Its hemodynamic effect leads to a decrease in post-load to the left ventricle, an increase in cardiac output, an increase in coronary diastolic perfusion pressure and a consequent improvement in coronary blood flow. Unlike inotropic agents and vasopressors, the benefit of IAB therapy occurs without increased myocardial oxygen consumption [5].
The IAB is indicated for patients with adjusted blood volume, using full inotropic doses (often with inotropic combination), and persisting with signs of poor tissue perfusion. It can be used as support until definitive therapy (myocardial revascularization, cardiac transplantation, for example), or as support until the resolution of the precipitating factors, surgical treatment of valvular heart disease or mechanical complications of AMI. Despite the routine use of IAB for decades in the treatment of AMI complicated with CS, it cannot be said that this strategy is associated with an improvement in the survival of these patients [23, 24].
The retrospective study of 223 patients with refractory heart failure who were treated with IAB at the Intensive Care Unit of the Heart Institute, HC-FMUSP, in the 5-year period, showed in 93.0% of the cases the low cardiac output. The IAB was used for an average of 19 days in patients with significant left ventricular dysfunction, with a mean ejection fraction of 24.0%. After 48 h of IAB, there was a significant improvement in the laboratory parameters of microhemodynamic evaluation, with reduction of serum lactate, increase of SvO2 and bicarbonate levels. IAB has been shown to be an important therapeutic intervention as a bridge for heart transplantation [25].
In patients who persist with tissue hypoperfusion despite the use of inotropics, vasodilators, IAB and myocardial revascularization procedure, when there is indication, there is the option of using another ventricular assist device, considered as a bridge for cardiac transplantation, for recovery, for therapeutic decision, or serve as a target therapy.
As the main cause of CS is AMI, the fundamental approach is rapid myocardial reperfusion therapy, either by angioplasty or via a surgical approach. This measure was well established in the SHOCK study [1]. In this study, patients less than 75 years of age who were within the first 36 h of AMI initiation and up to 18 h after onset of CS benefited from myocardial reperfusion therapy, with a 13.0% survival increase after 1-year of follow-up.
It is known that 40.0% of patients with post-infarction CS are older than 75 years. However, for this elderly population, there is controversy regarding the improvement of survival with early revascularization when compared to the conservative strategy of the initial clinical treatment.
In the SHOCK study, there was no difference in mortality in the subgroup of patients older than 75 years. Clinical judgment is important in the decision to indicate percutaneous coronary intervention in the elderly with AMI and CS. The presence of comorbidities with organic dysfunction should be considered such as chronic renal failure, heart failure, hematological diseases and dementias for better therapeutic decision [1, 2]. The choice between percutaneous angioplasty and myocardial revascularization surgery is a controversial issue in the case of CS. Surgery is the option in cases with mechanical complications (ventricular septal defect, acute mitral regurgitation and rupture of the left ventricular free wall) and in those with contraindication or failure of percutaneous treatment. However, the analysis of the SHOCK1 study showed a similar evolution among the groups submitted to revascularization, although the patients in the surgical group had a higher incidence of diabetes, coronary trunk lesion or triarterial lesions.
The goal of treatment of AMI complicated by CS is the rapid reestablishment of blood flow in the affected artery to improve the patient’s prognosis. The correction of hypoxemia and lactic acidosis, due to severe systemic hypoperfusion, is fundamental to prevent myocardial depression and reduction of response to vasopressors. Monitoring by pulmonary artery catheter (Swan-Ganz) and intra-arterial catheter is recommended to guide therapeutic interventions [29].
Volume replacement should be guided by pulmonary capillary pressure, arterial oxygen saturation, SBP and DC. Intravenous 0.9% NaCl, 250.0 mL, may be administered prior to right cardiac catheterization in case of suspected CS, after discarding pulmonary congestion and dyspnea. Excessive volume administration in the presence of extensive left ventricular AMI, especially in the elderly, may lead to acute pulmonary edema, but may be beneficial when the right ventricle is affected and the jugular venous pressure is not elevated [29].
| Conclusion | ▴Top |
Based on the literature review, the dosage of biomarkers in the daily clinical practice of the various cardiological centers will facilitate the diagnosis and the conduction of doubtful cases and the best evaluation of the degree of myocardial suffering after clinical events or surgical procedures, especially in cases of CS.
Conflict of Interest
There is no conflict of interest between authors.
| References | ▴Top |
- Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341(9):625-634.
doi pubmed - Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686-697.
doi pubmed - Dutt DP, Pinney SP. Clinical variability within the INTERMACS 1 profile: implications for treatment options. Curr Opin Cardiol. 2014;29(3):244-249.
doi pubmed - Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, et al. Interagency registry of mechanically assisted circulatory support. J Heart Lung Transplant. 2009;28(6):535-541.
doi pubmed - Hollenberg SM, Kavinsky CJ, Parrillo JE. Cardiogenic shock. Ann Intern Med. 1999;131(1):47-59.
doi pubmed - Avezum Junior Á, Feldman A, Carvalho AC, Sousa AC, Mansur Ade P, Bozza AE, Falcão Bde A, et al. V Diretriz da SociedadeBrasileira de Cardiologia Sobre o Tratamento do Infarto Agudo do Miocardio com Supradesnivel do Segmento ST. Arq Bras Cardiol. 2015;105(2 Suppl 1):1-105.
doi pubmed - Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, et al. Clinical features and outcomes of takotsubo (Stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
doi pubmed - Califf RM, Bengtson JR. Cardiogenic shock. N Engl J Med. 1994;330(24):1724-1730.
doi pubmed - Barry WL, Sarembock IJ. Cardiogenic shock: therapy and prevention. Clin Cardiol. 1998;21(2):72-80.
doi - Berger PB, Holmes DR, Jr., Stebbins AL, Bates ER, Califf RM, Topol EJ. Impact of an aggressive invasive catheterization and revascularization strategy on mortality in patients with cardiogenic shock in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial. An observational study. Circulation. 1997;96(1):122-127.
doi pubmed - Global Use of Strategies to Open Occluded Coronary Arteries I. A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med. 1997;337(16):1118-1123.
doi pubmed - Killip T, 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20(4):457-464.
doi - Kleine AH, Glatz JF, Van Nieuwenhoven FA, Van der Vusse GJ. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem. 1992;116(1-2):155-162.
pubmed - Pfisterer M. Right ventricular involvement in myocardial infarction and cardiogenic shock. Lancet. 2003;362(9381):392-394.
doi - Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow direct balloon-tipped catheter. N Engl J Med. 1970;283:441-451.
doi pubmed - Shah MR, O'Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141(4):528-535.
doi pubmed - Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, Pinsky MR, et al. Clinical review: Update on hemodynamic monitoring—a consensus of 16. Crit Care. 2011;15(4):229.
doi pubmed - Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184(5):514-520.
doi pubmed - Amaral AC, Park M. Monitorizacao do balanco entre oferta e consume de oxigenionasindrome do choque. Uma revisaosobresignificadofisiopatologico e clinico da saturacaovenosa central (ScvO2) e da saturacaomista de oxigenio (SvO2). Rev Bras Ter Intensiva. 2004;16(4):271-275.
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847.
doi pubmed - De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779-789.
doi pubmed - Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360(9328):196-202.
doi - Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287-1296.
doi pubmed - Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638-1645.
doi - Bezerra CG, Adam EL, Baptista ML, Ciambelli GS, Kopel L, Bernoche C, Lopes LN, et al. Aortic counterpulsation therapy in patients with advanced heart failure: analysis of the TBRIDGE registry. Arq Bras Cardiol. 2016 Jan;106(1):26-32.
doi pubmed - Sakai K, Nakagawa Y, Soga Y, Ando K, Yokoi H, Iwabuchi M, Yasumoto H, et al. Comparison of 30-day outcomes in patients <75 years of age versus >or=75 years of age with acute myocardial infarction treated by primary coronary angioplasty. Am J Cardiol. 2006;98(8):1018-1021.
doi pubmed - Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1607-1621.
doi - Godoy MF, Braile DM, Neto JP. A Troponina como Marcador de Injuria Celular Miocardica. Arq Bras Cardiol. 1998;71.
doi - Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107(24):2998-3002.
doi pubmed - Mair J, Wagner I, Jakob G, Lechleitner P, Dienstl F, Puschendorf B, Michel G. Different time courses of cardiac contractile proteins after acute myocardial infarction. Clin Chim Acta. 1994;231(1):47-60.
doi - Guest TM, Ramanathan AV, Tuteur PG, Schechtman KB, Ladenson JH, Jaffe AS. Myocardial injury in critically ill patients. A frequently unrecognized complication. JAMA. 1995;273(24):1945-1949.
doi pubmed - Jaffe AS, Landt Y, Parvin CA, Abendschein DR, Geltman EM, Ladenson JH. Comparative sensitivity of cardiac troponin I and lactate dehydrogenase isoenzymes for diagnosing acute myocardial infarction. Clin Chem. 1996;42(11):1770-1776.
pubmed - Bertinchant JP, Larue C, Pernel I, et al. Interet du dosage de la troponine humaine dans le diagnostic de l'infarction aigu du myocarde. Arch Mal Couer Vaiss. 1996;89:63-68.
pubmed - Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997;337(23):1648-1653.
doi pubmed - Luscher MS, Thygesen K, Ravkilde J, Heickendorff L. Applicability of cardiac troponin T and I for early risk stratification in unstable coronary artery disease. TRIM Study Group. Thrombin Inhibition in Myocardial ischemia. Circulation. 1997;96(8):2578-2585.
doi pubmed - Wu AH, Feng YJ, Contois JH, Pervaiz S. Comparison of myoglobin, creatine kinase-MB, and cardiac troponin I for diagnosis of acute myocardial infarction. Ann Clin Lab Sci. 1996;26(4):291-300.
pubmed - Joris PJ, Plat J, Bakker SJ, Mensink RP. Effects of long-term magnesium supplementation on endothelial function and cardiometabolic risk markers: A randomized controlled trial in overweight/obese adults. Sci Rep. 2017;7(1):106.
doi pubmed - Baker WL. Treating arrhythmias with adjunctive magnesium: identifying future research directions. Eur Heart J Cardiovasc Pharmacother. 2017;3(2):108-117.
pubmed - Yu L, Li H, Wang SX. Serum Magnesium and Mortality in Maintenance Hemodialysis Patients. Blood Purif. 2017;43(1-3):31-36.
doi pubmed - Fang X, Wang K, Han D, He X, Wei J, Zhao L, Imam MU, et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med. 2016;14(1):210.
doi pubmed - van Mil AC, Greyling A, Zock PL, Geleijnse JM, Hopman MT, Mensink RP, Reesink KD, et al. Impact of volunteer-related and methodology-related factors on the reproducibility of brachial artery flow-mediated vasodilation: analysis of 672 individual repeated measurements. J Hypertens. 2016;34(9):1738-1745.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


