| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website http://www.cardiologyres.org |
Original Article
Volume 8, Number 5, October 2017, pages 206-213
Association of Heart Rate Recovery With Microalbuminuria in Non-Obstructive Coronary Artery Disease
Mustafa Yurtdasa, d, Mahmut Ozdemirb, Nesim Aladagb, Yalin Tolga Yaylalic
aDepartment of Cardiology, Balikesir Sevgi Hospital, Balikesir, Turkey
bDepartment of Cardiology, Van Education and Research Hospital, Van, Turkey
cDepartment of Cardiology, School of Medicine, Pamukkale University, Denizli, Turkey
dCorresponding Author: Mustafa Yurtdas, Department of Cardiology, Sevgi Hospital, Pasaalani Mah. 10020, Balikesir, Turkey
Manuscript submitted August 14, 2017, accepted August 28, 2017
Short title: Heart Rate Recovery and Microalbuminuria
doi: https://doi.org/10.14740/cr593w
| Abstract | ▴Top |
Background: Non-obstructive coronary artery disease (CAD) is associated with significantly increased risk for myocardial infarction. Heart rate recovery (HRR), a measure of autonomic function, is a strong predictor of all-cause mortality. Microalbuminuria, a marker of early arterial disease, is an independent risk factor for cardiovascular disease and mortality. We aimed to investigate HRR and determine its relationship with microalbuminuria in patients with non-obstructive CAD.
Methods: We prospectively studied 565 patients who underwent elective coronary angiography. All participants underwent urinary analysis and then an exercise test. Microalbuminuria was defined as an urinary albumin-to-creatinine ratio (UACR) of 30 - 299 mg/g. The HRR was abnormal if ≤ 12 beats/min during the first minute after exercise. First, all patients were divided into two groups, patients with microalbuminuria (n = 152) and patients without microalbuminuria (n = 413). Then, all patients were re-divided into two groups, those with lower HRR (≤ 12 beats/min, n = 126) and those with higher HRR (> 12 beats/min, n = 439).
Results: Patients with microalbuminuria had lower HRR and patients with lower HRR had higher UACR. While UACR was negatively correlated with HRR in patients with microalbuminuria (r = -0.424; P < 0.001) and in patients with lower HRR (r = -0.192; P= 0.042), there was no correlation of UACR with HRR in neither patients with normoalbuminuria nor patients with higher HRR, respectively. In the all study population, there was a significant inverse association between UACR and HRR (r = -0.445, P < 0.001), and UACR independently predicted the presence of lower HRR (P < 0.001).
Conclusions: Our findings showed that there was a significant inverse association between UACR and HRR in patients especially with microalbuminuria, and that albuminuria might predict cardiac autonomic imbalance evaluated by HRR in patients with non-obstructive CAD.
Keywords: Heart rate recovery; Microalbuminuria; Non-obstructive; Coronary artery disease
| Introduction | ▴Top |
Coronary artery disease (CAD) is a chronic and progressive disease. Clinically significant CAD (defined as one or more lesions with ≥ 50% stenosis in the left main or ≥ 70% stenosis in any other epicardial coronary artery on coronary angiogram) is often symptomatic. Non-obstructive CAD has a long asymptomatic phase, is usually left untreated, but it could culminate in acute clinical coronary events just like obstructive CAD [1-3].
Microalbuminuria, described as small quantities of albumin in the urine ranging between 30 and 300 mg/dL per day (20 - 200 mg/min), is an easily measurable parameter. Microalbuminuria reflects vascular damage and appears to be a marker of early arterial disease and endothelial dysfunction [4]. Increased urinary albumin excretion is a powerful predictor of cardiovascular disease and mortality in diabetic, hypertensive and elderly individuals, and in the apparently healthy general population [5-8].
Heart rate recovery (HRR) after exercise test, as a simple method for evaluating the effects of autonomic function on the cardiovascular system, has been shown to be affected in patients with CAD [9-13]. HRR has recently emerged as a powerful predictor of mortality [12, 13]. The predictive ability of HRR has been shown to be independent of the angiographic severity of coronary disease [13].
There have been insufficient data about non-obstructive CAD and its outcomes. To determine which individuals with non-obstructive CAD are at low or high risk for developing cardiac events may be of clinical importance. The use of micoalbuminuria had been recommended in hypertensive patients with or without diabetes mellitus [14]. A better understanding of the relationship of HRR with microalbuminuria could help better risk stratify those patients at high risk of coronary events.
We hypothesized that abnormal HRR might be related to microalbuminuria in non-obstructive CAD. Therefore, we aimed to evaluate HRR, and determine its relationship with microalbuminuria in patients with non-obstructive CAD.
| Materials and Methods | ▴Top |
The study design was prospective. The study included 565 consecutive patients who underwent an elective coronary angiography due to the suspicion of CAD from August 2014 to December 2016. All participants had a thorough medical history, physical examination, and electrocardiographic, echocardiographic, angiographic evaluations and laboratory analyses. Exclusion criteria were diabetes mellitus (defined as fasting plasma glucose ≥ 126 mg/dL or 2-h plasma glucose ≥ 200 mg/dL or the use of antidiabetic drugs), hypertension (defined as blood pressure of ≥ 140/90 mm Hg or the use of antihypertensive drugs), heavy smoking (described as smoking ≥ 20 cigarettes per day), reduced renal function (serum creatinine level ≥ 1.5 mg/dL) or renal diseases, macroalbuminuria (defined as an urinary albumin excretion rate ≥ 300 mg/day), hepatic disease, aortic disease, peripheral arterial disease, congenital heart disease, ventricular hypertrophy, slow coronary flow, previous coronary or valvular intervention and/or cardiac surgery, myocardial disease, systolic dysfunction (ejection fraction < 50%), digoxin use, left bundle branch block, cardiac arrhythmias, moderate or severe (> 2+) valvular regurgitation or any valvular stenosis, thyroid disorders, recent invasive surgery, obesity (body mass index > 30 kg/m2), the use of any anti-inflammatory medication, autoimmune and inflammatory diseases with clinical or biochemical evidence. We also excluded patients who had contraindications to exercise testing, and failed to reach 85% of their age-predicted maximal heart rate during exercise test. All participants gave the written informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Pamukkale University Medical Ethics Committee (registration number: 60116787-020/39556, date of issue: July 11, 2014).
Laboratory analyses
All blood samples were drawn after a 12-h overnight fasting for total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), complete blood count (CBC), glucose and creatinine. Assays for cholesterol profile and creatinine were carried out using a Cobas Integra 800 automated analyzer (Roche Diagnostics, Mannheim, Germany). The glomerular filtration rate (GFR) was calculated as a function of age, serum creatinine, and race using the simplified modified diet in renal disease (MDRD) equation (eGFR (mL/min/1.73 m2) = 186.3 × creatinine-1.154 × age-0.203 × (0.742 if female)). High-sensitivity C-reactive protein (hsCRP) was analyzed using a method of latex turbidimetric immunoassay, with the minimum limit of detection at 0.15 mg/L (Cobas C Systems, Roche-Hitachi, Rotkreuz, Switzerland). The hsCRP less than 3 mg/L was accepted as normal.
Coronary angiography
Coronary angiographies were performed with a femoral or radial approach using the standard Judkins technique through a 6-F introducer sheath. Consistent with the current literature, non-obstructive CAD was defined as a coronary artery stenosis 20% or greater but less than 50% in the left main coronary artery (LMCA) or a stenosis 20% or greater but less than 70% in any other epicardial coronary artery [2, 3]. The extent and severity of CAD were evaluated by the Gensini score [15], which is based on the percentage of luminal narrowing (1-25%: 1 point; 26-50%: 2 points; 51-75%: 4 points; 76-90%: 8 points; 91-99%: 16 points, and 100%: 32 points). This score was then multiplied by a factor according to the functional importance of the myocardial area supplied by that segment. The multiplication factor is 5 for the LMCA; 2.5 for proximal left anterior descending coronary artery (LAD); 2.5 for proximal circumflex artery (CX); 1.5 for mid-LAD, and 1 for distal LAD, and mid/distal CX artery, and all segments of the right coronary artery; and 0.5 for other coronary segments. Finally, the Gensini score was obtained by summation of individual coronary segment scores. Furthermore, the number of diseased coronary arteries (DCA) was represented as one-, two- and three-vessel involvement. Gensini score and the number of DCA were recorded by a cardiologist who was blinded to study grouping, exercise and laboratory findings.
Urinary analyses
Urinary tests were done at the end of second week after coronary angiography. Early morning midstream urine sample was collected and analyzed. Urinary creatinine was measured using a modified Jaffe method. Urinary albumin was determined using an immunonephelometry method. Microalbuminuria was accepted as persistent if the urinary albumin-to-creatinine ratio (UACR) was in the range of 30 - 299 mg/g in at least two of three consecutive samples [4].
Heart rate recovery and Duke treadmill score
Within 2 days after completion of urinary analyses, all participants underwent the standard or symptom-limited treadmill test, with a goal of achieving at least 85% of the age-predicted maximum heart rate (220 - age). Certain medications such as beta-blocker and calcium channel antagonists that may affect exercise test results were discontinued for 72 h prior to the test. Participants were encouraged to exercise until they experienced limiting symptoms. During each exercise stage and every minute for 3 min after recovery, heart rate, cardiac rhythm, and blood pressure were recorded. After peak exercise, subjects walked a 2-min cool-down period at a speed of 1.5 mph and a grade of 2.5%. HRR was described as the change in heart rate from peak exercise to 1 min after exercise. HRR of 12 beats/min or less was defined as abnormal [9-11]. In addition, the Duke treadmill score (DTS) was calculated for each participant according to the following formula: DTS = (exercise duration in minutes) - (5 × ST deviation in millimeters) - (4 × treadmill angina index). The treadmill angina index is equal to 0 for no exercise angina, 1 for exercise-non-limiting angina, and 2 for exercise-limiting angina [16, 17]. HRR and DTS were recorded by another cardiologist who was blinded to study grouping, angiographic and laboratory findings.
Statistical methods
The statistical analyses were done using the SPSS software package (version 20.0; IBM, SPSS Inc., Chicago, IL, USA). All enrolled patients were divided into two groups based on microalbuminuria: non-obstructive CAD patients with microalbuminuria (n = 152) vs. non-obstructive CAD patients without microalbuminuria (n = 413). Categorical variables are shown as frequencies and percentage values, and comparisons were made using a Chi-square test or Fisher’s exact test. The distribution of data was checked with Kolmogorov-Smirnov test. Continuous variables are shown as mean ± SD, and were compared between groups using independent t-test (for normally distributed variables) or a Mann-Whitney U test (for variables not normally distributed). Pearson’s or the Spearman correlation analysis was used to detect the relationship between variables. To describe the independent predictors of the presence of lower HRR value (≤ 12 beats/min), all study participants were subsequently stratified into the two groups according to the values of HRR (≤ 12 beats/min, n = 126 vs. > 12 beats/min, n = 439). All variables with a P value of < 0.05 in the univariate analysis were entered into the multivariate logistic regression analysis. The variables “one-vessel, two-vessel and three-vessel involvement” were not included as a covariate in the multivariate analysis because of collinearity with the variable “the number of diseased coronary arteries”, which was used instead. The results are shown as odds ratio (OR) with 95% confidence interval (CI). Receiver operating characteristic (ROC) analysis was constructed to calculate the area under the curve (AUC) and define the best cutoff points of UACR to predict the lower values of HRR (≤ 12 beats/min), with the maximal sensitivity and specificity. The differences with probability values (P) of less than 0.05 were considered as significant.
| Results | ▴Top |
A total of 565 patients (349 male, 61.8%) were enrolled. All patients had non-obstructive CAD. The mean age was 54 ± 7 years, the mean UACR was 31 ± 40 mg/g, and the mean HRR was 21 ± 9 beats/min. In the all study population, there was a significant inverse association between UACR and HRR (r = -0.445, P < 0.001). Baseline clinical, demographic, and laboratory characteristics were compared between non-obstructive CAD patients with and without microalbuminuria (Table 1). The two groups were similar regarding age, sex, family history (FH), ejection fraction (EF), glucose, lipid profile, creatinine, eGFR, body mass index (BMI), and baseline heart rate. Patients with microalbuminuria had higher systolic blood pressure (SBP), diastolic blood pressure (DBP), UACR and hsCRP. Patients with microalbuminuria had also greater Gensini score and the number of DCA. Maximum heart rate, and heart rate at first minute after exercise were significantly greater in patients with microalbumuria than in patients without microalbumuria. HRR and DTS were significantly lower in patients with microalbumuria than in patients without microalbumuria. In patients with microalbumuria, UACR was positively correlated with hsCRP (r = 0.426; P < 0.001), Gensini score (r = 0.423; P < 0.001) and the number of DCA (r = 0.247; P < 0.001), and negatively correlated with HRR (r = -0.424; P < 0.001) and DTS (r = -0.334; P < 0.001). In addition, hsCRP was negatively correlated with HRR (r = -0.360; P < 0.001) and DTS (r = -0.319; P < 0.001) in patients with microalbumuria. There were no associations among the above-mentioned variables in patients without microalbumuria. Table 2 shows the clinical, demographic, and laboratory data of each subgroup after grouping by HRR values (≤ 12 beats/min vs. > 12 beats/min). There were no statistically significant differences in terms of gender, FH, EF, SBP, DBP, BMI, creatinine, eGFR and baseline heart rate between patients with lower (≤ 12 beats/min) and higher (> 12 beats/min) HRR values. Patients with abnormal or lower HRR tended to be older and had higher LDL, triglyceride and glucose levels. UACR, hsCRP, Gensini score and the number of DCA were higher, and DTS was lower in patients with lower HRR. UACR was positively correlated with hsCRP (r = 0.180; P = 0.044), Gensini score (r = 0.451; P < 0.001) and the number of DCA (r= 0.279; P = 0.002), and negatively correlated with HRR (r = -0.192; P = 0.042) and DTS (r = -0.212; P = 0.017); moreover, hsCRP was negatively correlated with HRR (r = -0.187; P = 0.034) and DTS (r = -0.326; P < 0.001) in patients with lower HRR. We did not observe any associations among the above remarked variables in patients with higher HRR. Table 3 shows the results of univariate and multivariate analyses to define the independent predictors of the presence of lower HRR in all patients with non-obstructive CAD. UACR and hsCRP were independently associated with the presence of lower HRR (UACR: OR: 0.988; 95% CI: 0.983 - 0.993; P < 0.001 and hsCRP: OR: 0.807; 95% CI: 0.735 - 0.886; P < 0.001). Figure 1 displays ROC curve analysis of UACR for predicting lower HRR value in all patients with non-obstructive CAD. This analysis indicated that an UACR of 9.7 mg/g or higher predicted the presence of a lower HRR with a maximal sensitivity of 71% and specificity of 58% (AUC: 0.68; CI: 0.62 - 0.74; P < 0.001).
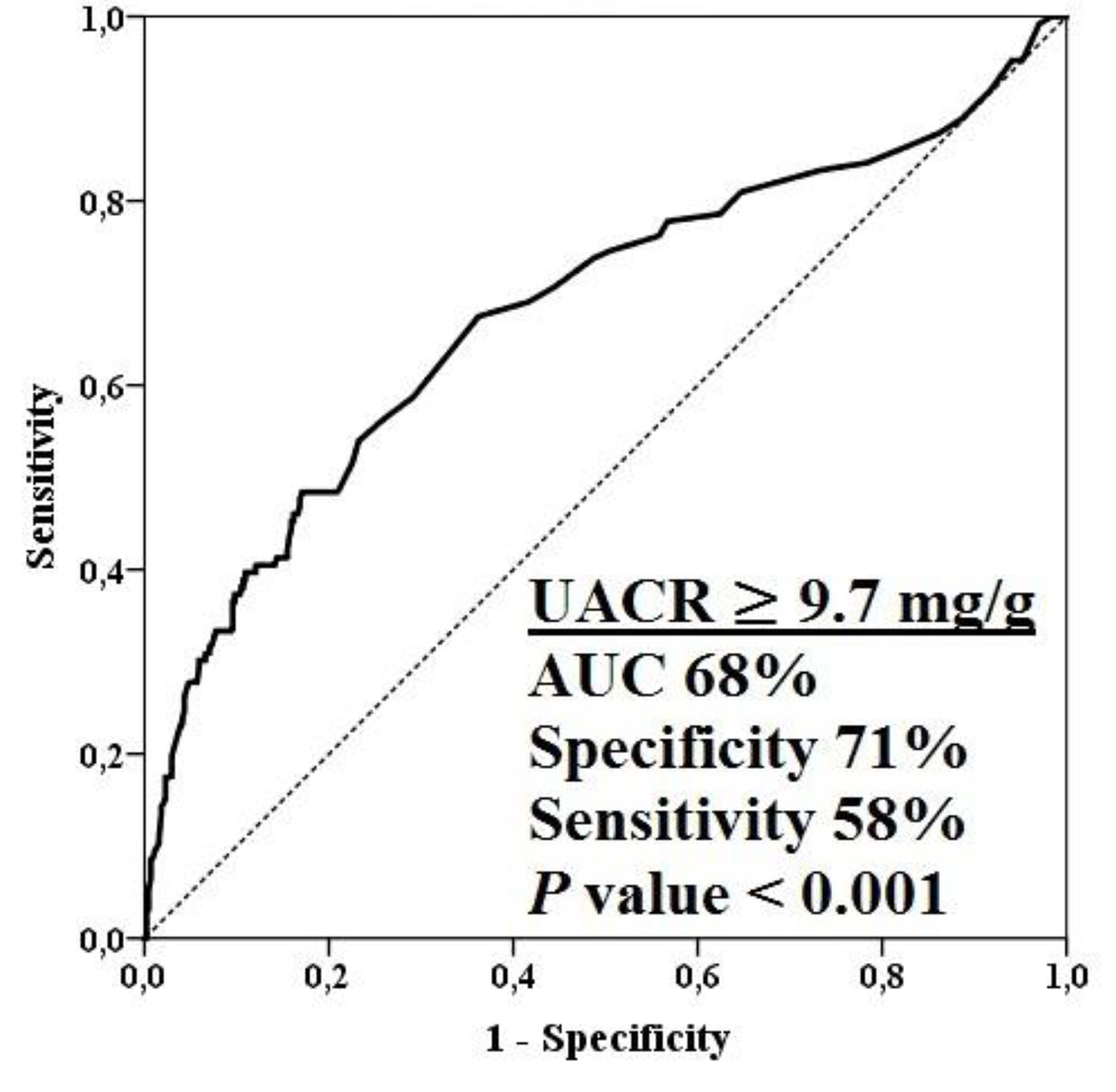 Click for large image | Figure 1. ROC curve analysis of UACR for predicting lower HRR value in all patients with non-obstructive CAD. CAD: coronary artery disease; HRR: heart rate recovery; UACR: urinary albumin-to-creatinine ratio. |
 Click to view | Table 1. Baseline Clinical, Demographic and Laboratory Characteristics of Non-Obstructive Coronary Artery Disase Patients With and Without Microalbuminuria |
 Click to view | Table 2. Baseline Clinical, Demographic and Laboratory Characteristics of the Patients According to the Values of HRR (≤ 12 beats/min vs. > 12 beats/min) |
 Click to view | Table 3. Independent Predictors of the Presence of Lower HRR Value (≤ 12 beats/min) in Patients With Non-Obstructive Coronary Artery Disease |
| Discussion | ▴Top |
Our results revealed that greater autonomic dsyfunction (lower HRR) was observed in patients with microalbuminuria than patients without microalbuminuria. Moreover, the key finding of our study was that while UACR was negatively correlated with HRR in patients with microalbuminuria, it was not correlated with HRR in patients without microalbuminuria, suggesting that albumin excretion could be related to cardiac autonomic dysfunction in this group of patients. To our knowledge, this is the first report demonstrating an important association between microalbuminuria and abnormal HRR after exercise in patients with non-obstructive CAD.
In this study, diabetic, hypertensive and obese patients were excluded in order to eliminate their potential confounding effects on albuminuria and autonomic function. In addition, the levels of blood pressure in the groups were within the normotensive range. Therefore, this study may not suggest diabetes, hypertension, and obesity as the factors playing role in the association of microalbuminuria and lower HRR. Several reasons may explain this association in non-obstructive CAD patients. First, albuminuria might be associated with the extent and severity of the underlying coronary disease. We observed that patients with microalbuminuria had higher Gensini score and more multi-vessel disease than those without microalbuminuria, and that there was a strong association between Gensini score and UACR in patients with microalbuminuria, but not in patients without microalbuminuria. Hoseini et al showed that the patients with microalbuminuria had much greater atherosclerotic burden in the form of multi-vessel disease than those without microalbuminuria [18]. In another study, Rein et al investigated whether albuminuria was associated with ≥ 50% coronary artery stenosis and they showed that albuminuria was strongly associated with angiographically detected coronary atherosclerosis in 914 patients with or without type 2 diabetes mellitus, and they also found that the prevalence of stenoses ≥ 50% increased significantly from patients with normoalbuminuria over those with microalbuminuria to those with macroalbuminuria [19]. These findings are in line with the current study. Although abnormal HRR was not helpful in determining the presence of the underlying significant CAD, it might be associated with the greater extent of CAD [13]. We found that patients with abnormal HRR had higher Gensini score and more multi-vessel disease than patients with normal HRR values. In a study by Ghaffari et al, patients with abnormal HRR were more likely to have more severe coronary disease than those with normal HRR [10]. We also found that both patients with abnormal HRR and patients with microalbuminuria had higher UACR and less DTS score than those of counterparts, indicating that both cardiac autonomic dysfunction and microalbuminuria make these patients more prone to myocardial ischemia during exercise stress test. A previous study showed that patients with abnormal HRR had a greater degree of myocardial ischemia on myocardial scintigraphy during exercise [9]. Very recently, Osugi et al assessed the impact of albuminuria on the incidence of periprocedural myocardial injury in patients who underwent elective percutaneous coronary intervention, and showed that patients with albuminuria had a 4.2-fold higher risk for periprocedural myocardial infarction than did patients with normoalbuminuria [20]. All these data could suggest that both albuminuria and abnormal HRR in non-obstructive CAD patients might share common pathophysiologic pathway, consisting of endothelial damage, high atherosclerotic and thus, ischemic burden, which was closely associated with cardiac autonomic impairment.
Second, the underlying chronic inflammation might be responsible for both microalbuminuria and abnormal HRR in this patient population. We observed that patients with microalbuminuria had greater hsCRP values than patients without microalbuminuria. Another important finding in our study was that patients with abnormal HRR had greater hsCRP values than patients with normal HRR. Moreover, we found that UACR and hsCRP were independent predictors of the presence of abnormal HRR in non-obstructive CAD patients, suggesting that albumin excretion and systemic inflammation and their interaction could have significant effects on autonomic dysfunction. Elevations of inflammatory mediators might directly alter glomerular function and thus, might be causally involved in the development of microalbuminuria. Serum hsCRP has been associated with vascular inflammation, and significantly correlated with microalbuminuria in the general population [21]. In the former studies, urinary albumin excretion has been shown to increase in diabetic and non-diabetic patients [5-8], inflammatory diseases [22, 23], and acute myocardial infarction as well [24]. A growing number of evidence in recent years has demonstrated that cardiac autonomic nervous activity, as evaluated by heart rate variability (HRV) and HRR, is associated with inflammatory markers [25, 26]. More recently, we have shown that impaired cardiac autonomic function, as evidenced by a slower HRR, is closely related to inflammatory markers such as hsCRP in both CAD and cardiac syndrome X [11].
Third, cardiac autonomic dysfunction may play a direct causative role in renal disease beacuse the renal vasculature is extensively innervated by the sympathetic nervous system [27]. Although we did not search such a causality between albuminuria and autonomic function in our study, our findings could still be useful in understanding why albuminuria was associated with abnormal HRR. Moran et al conducted a study on whether cardiovascular autonomic neuropathy, assessed by HRV, was related to microalbuminuria in young and middle-aged patients with type 2 diabetes, and they found that cardiac autonomic dysfunction was independently associated with increased albuminuria [28]. Recently, Maguire et al showed that abnormal pupillary response, a marker of autonomic dysfunction, preceded the development of microalbuminuria and diabetic retinopathy 12 years later [29]. These results could suggest that damage to the autonomic nerves purveying the renal vasculature might pave the way for increased renal blood flow, glomerular hyperfiltration, and, thus, microalbuminuria, as suggested previously by some authors.
Study strengths and limitations
The strengths of the present study were as follows: first, its large sample size; second, the careful selection and the angiographical characterization of the recruited participants; third, the use of at least two morning urine samples to determine the presence of albuminuria. However, it had several limitations. First, since the population studied was highly selected, our results might not be applicable to other populations; second, our study had cross-sectional design without long-term follow-up data, not allowing to define causality. Lastly, we did not specifically carry out intravascular studies to fully define the degree of coronary stenosis or characterize the plaque burden.
Conclusion
We found that non-obstructive CAD patients with microalbuminuria had lower HRR value, and non-obstructive CAD patients with abnormal HRR had higher UACR when compared to counterparts. We also found that there was a significant inverse association between UACR and HRR in patients particularly with microalbuminuria, and that albuminuria might predict cardiac autonomic imbalance assessed by HRR in patients with non-obstructive CAD. If our findings can be confirmed by prospective randomized large-scale studies, the routine measurement and detection of these high risk parameters (microalbuminuria and abnormal HRR after exercise) in this patient population could be applied into clinical practice.
Funding Sources
There are no external funding sources for this manuscript.
Conflict of Interest
There is no potential conflict of interest related to this manuscript.
| References | ▴Top |
- Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949-3003.
doi pubmed - Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754-1763.
doi pubmed - Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66(17):1918-1933.
doi pubmed - de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17(8):2100-2105.
doi pubmed - Sukhija R, Aronow WS, Kakar P, Garza L, Sachdeva R, Sinha A, Mehta JL. Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitus. Am J Cardiol. 2006;98(3):279-281.
doi pubmed - Bigazzi R, Bianchi S, Baldari D, Campese VM. Microalbuminuria predicts cardiovascular events and renal insufficiency in patients with essential hypertension. J Hypertens. 1998;16(9):1325-1333.
doi pubmed - Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction - the Hoorn Study. Kidney Int Suppl. 2004;92:S42-44.
doi pubmed - Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777-1782.
doi pubmed - Georgoulias P, Orfanakis A, Demakopoulos N, Xaplanteris P, Mortzos G, Vardas P, Karkavitsas N. Abnormal heart rate recovery immediately after treadmill testing: correlation with clinical, exercise testing, and myocardial perfusion parameters. J Nucl Cardiol. 2003;10(5):498-505.
doi - Ghaffari S, Kazemi B, Aliakbarzadeh P. Abnormal heart rate recovery after exercise predicts coronary artery disease severity. Cardiol J. 2011;18(1):47-54.
pubmed - Yurtdas M, Yaylali YT, Aladag N, Ozdemir M, Ceylan Y, Gencaslan M, Akbulut T. Heart rate recovery after exercise and its relation with neutrophil-to-lymphocyte ratio in patients with cardiac syndrome X. Coron Artery Dis. 2014;25(6):485-492.
doi pubmed - Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351-1357.
doi pubmed - Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42(5):831-838.
doi - Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219.
doi pubmed - Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606.
doi - Mark DB, Hlatky MA, Harrell FE, Jr., Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793-800.
doi pubmed - Shaw LJ, Peterson ED, Shaw LK, Kesler KL, DeLong ER, Harrell FE, Jr., Muhlbaier LH, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98(16):1622-1630.
doi pubmed - Hoseini VN, Rasouli M. Microalbuminuria correlates with the prevalence and severity of coronary artery disease in non-diabetic patients. Cardiol J. 2009;16(2):142-145.
pubmed - Rein P, Vonbank A, Saely CH, Beer S, Jankovic V, Boehnel C, Breuss J, et al. Relation of albuminuria to angiographically determined coronary arterial narrowing in patients with and without type 2 diabetes mellitus and stable or suspected coronary artery disease. Am J Cardiol. 2011;107(8):1144-1148.
doi pubmed - Osugi N, Suzuki S, Ishii H, Yasuda Y, Shibata Y, Tatami Y, Ota T, et al. Impact of albuminuria on the incidence of periprocedural myocardial injury in patients undergoing elective coronary stent implantation. Am J Cardiol. 2014;114(1):42-46.
doi pubmed - Stuveling EM, Bakker SJ, Hillege HL, Burgerhof JG, de Jong PE, Gans RO, de Zeeuw D, et al. C-reactive protein modifies the relationship between blood pressure and microalbuminuria. Hypertension. 2004;43(4):791-796.
doi pubmed - Batlle-Gualda E, Martinez AC, Guerra RA, Pascual E. Urinary albumin excretion in patients with systemic lupus erythematosus without renal disease. Ann Rheum Dis. 1997;56(6):386-389.
doi pubmed - Mahmud N, O’Connell MA, Stinson J, Goggins MG, Weir DG, Kelleher D. Tumour necrosis factor-alpha and microalbuminuria in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7(3):215-219.
pubmed - Ota H, Takeuchi T, Sato N, Hasebe N. Dipstick proteinuria as a surrogate marker of long-term mortality after acute myocardial infarction. J Cardiol. 2013;62(5):277-282.
doi pubmed - Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25(5):363-370.
doi pubmed - Jae SY, Ahn ES, Heffernan KS, Woods JA, Lee MK, Park WH, Fernhall B. Relation of heart rate recovery after exercise to C-reactive protein and white blood cell count. Am J Cardiol. 2007;99(5):707-710.
doi pubmed - Johns EJ. The physiology and pharmacology of the renal nerves. Pol Arch Med Wewn. 1991;85(3):141-149.
pubmed - Moran A, Palmas W, Field L, Bhattarai J, Schwartz JE, Weinstock RS, Shea S. Cardiovascular autonomic neuropathy is associated with microalbuminuria in older patients with type 2 diabetes. Diabetes Care. 2004;27(4):972-977.
doi pubmed - Maguire AM, Craig ME, Craighead A, Chan AK, Cusumano JM, Hing SJ, Silink M, et al. Autonomic nerve testing predicts the development of complications: a 12-year follow-up study. Diabetes Care. 2007;30(1):77-82.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.


