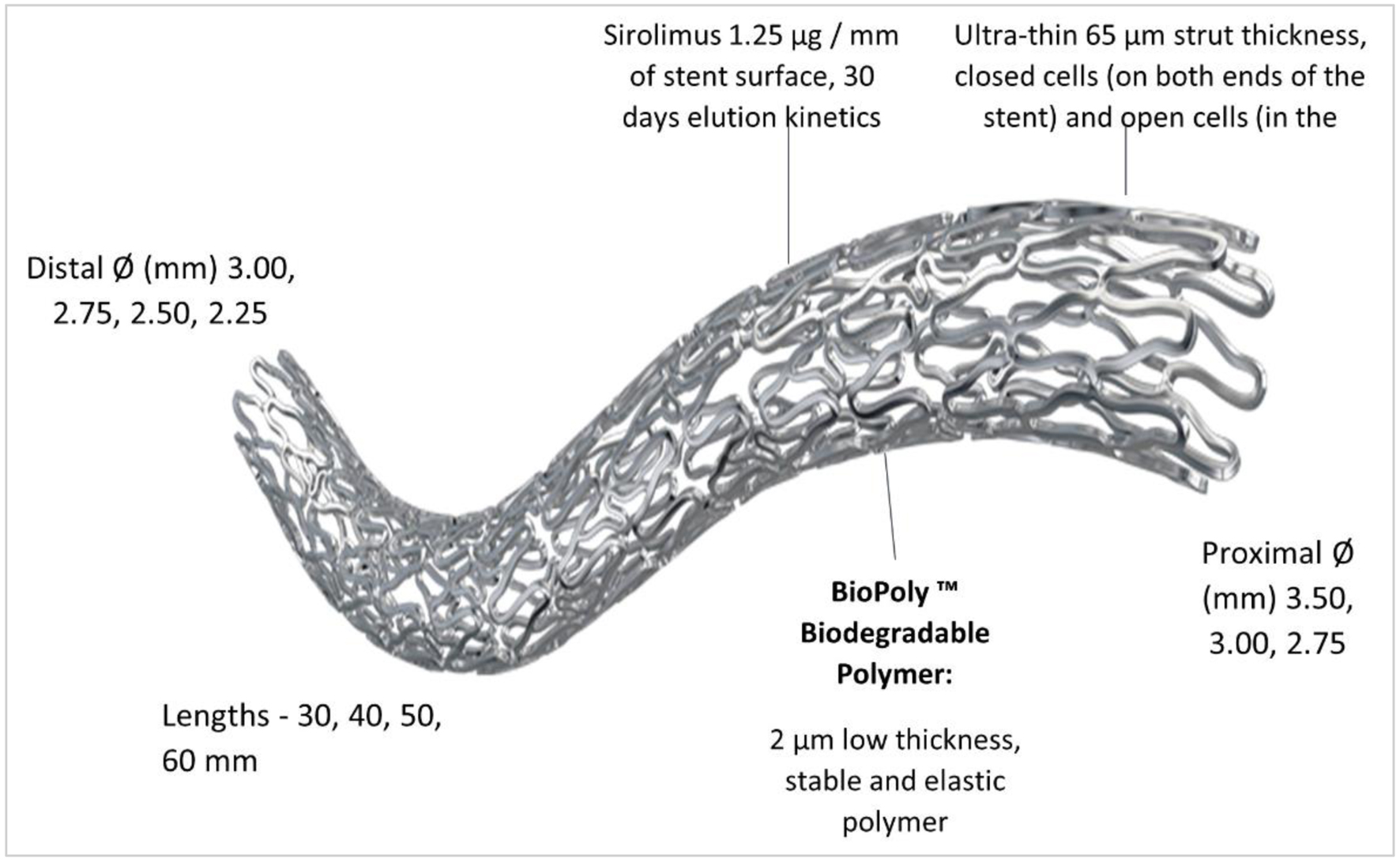
Figure 1. Design and features of BioMime™ Morph SES. SES: sirolimus-eluting stent.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 15, Number 3, June 2024, pages 169-178
Long-Term Safety and Performance of BioMime™ Morph Sirolimus-Eluting Coronary Stent System for Very Long Coronary Lesions
Figures

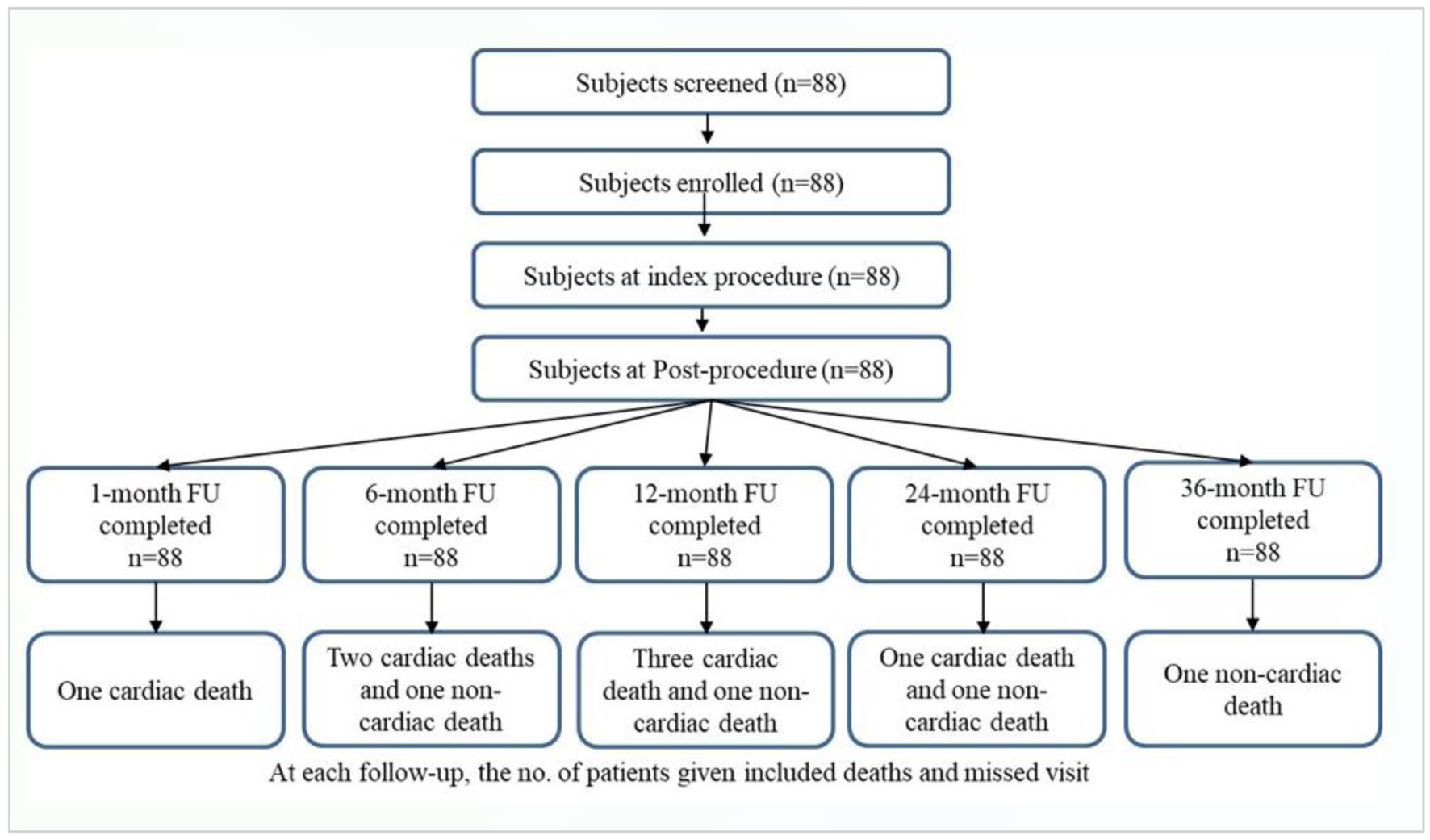
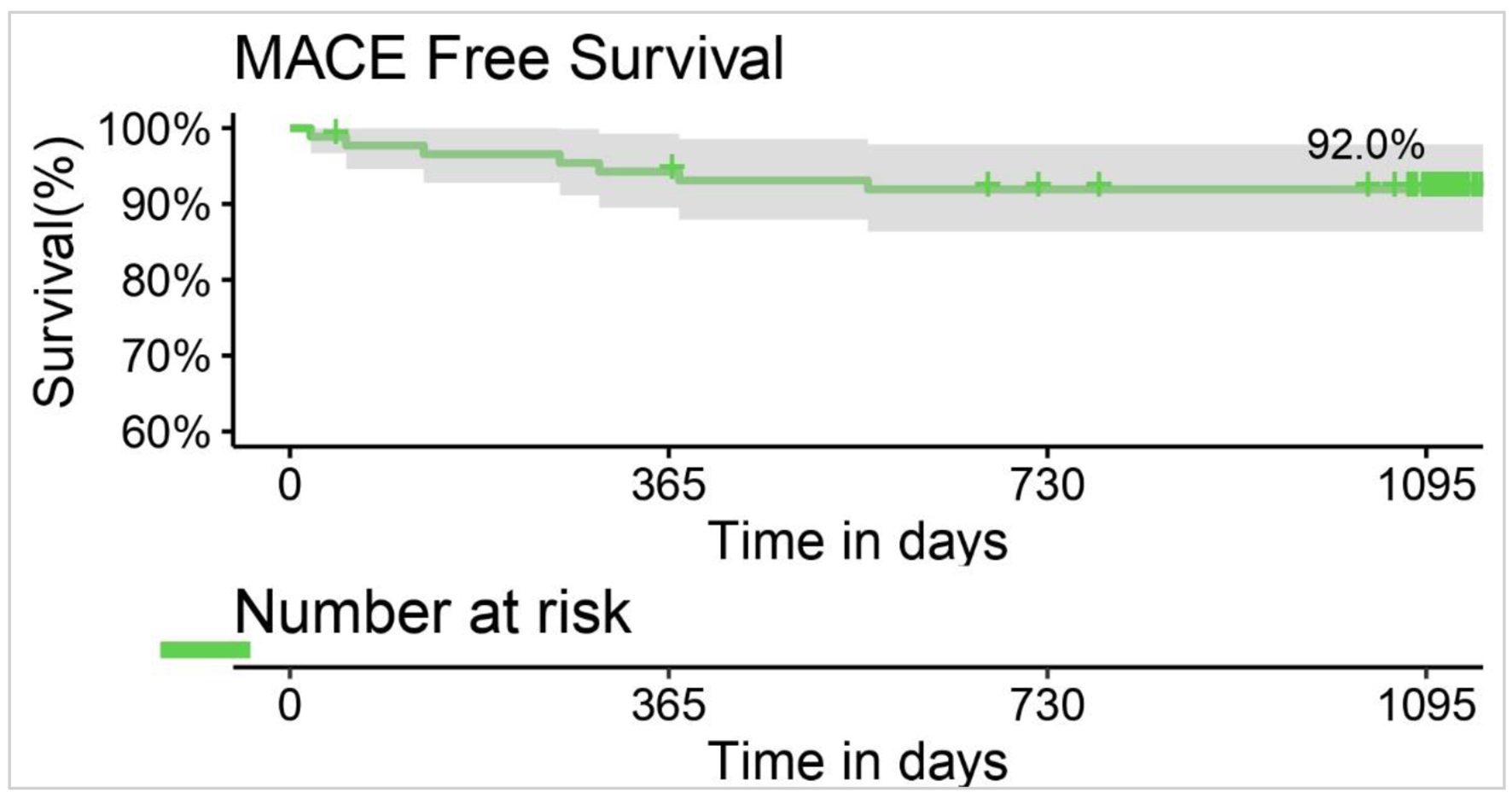
Tables
| Characteristics | n = 88 |
|---|---|
| BMI: body mass index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-elevation myocardial infarction. | |
| Age, years, mean ± SD | 58.72 ± 10.10 |
| Sex, n (%) | |
| Male | 73 (82.95) |
| Female | 15 (17.05) |
| BMI, kg/m2, mean ± SD | 25.59 ± 3.35 |
| Systolic blood pressure, mm Hg, mean ± SD | 128.91 ± 23.78 |
| Diastolic blood pressure, mm Hg, mean ± SD | 80.94 ± 10.03 |
| Heart rate, beats per minute, mean ± SD | 77.32 ± 10.81 |
| Medical history, n (%) | |
| Diabetes mellitus | 52 (59.09) |
| Hypertension | 48 (54.55) |
| Dyslipidemia | 1 (1.14) |
| Smoking | 14 (15.91) |
| Alcohol consumption | 6 (6.82) |
| COPD | 2 (2.27) |
| Ischemic heart disease | 69 (78.41) |
| Angina | 72 (81.82) |
| PCI | 2 (2.27) |
| Stroke | 2 (2.27) |
| Other illness | 1 (1.14) |
| Family history of CAD | 4 (4.55) |
| LVEF, %, mean ± SD | 50.23 ± 9.22 |
| Cardiac status, n (%) | |
| Stable angina | 2 (2.27) |
| Unstable angina | 11 (12.50) |
| STEMI | 44 (50.00) |
| NSTEMI | 5 (5.68) |
| Asymptomatic/silent ischemia | 2 (2.27) |
| Variables | Treated lesions (n = 92)/patients (n = 88) |
|---|---|
| CASS: coronary artery surgery study; CTO: chronic total occlusion; LAD: left anterior descending artery; LCx: left circumflex artery; RCA: right coronary artery. | |
| Diseased vessel, n (%) | |
| Single vessel | 29 (32.95) |
| Double vessel | 41 (46.6) |
| Triple vessel or more | 18 (20.45) |
| Lesion type, n (%) | |
| Calcified | 4 (4.35) |
| Diffused | 1 (1.09) |
| CTO | 5 (5.43) |
| Thrombus | 17 (18.48) |
| Discrete | 11 (11.96) |
| Tandem | 1 (1.09) |
| Lesion location (CASS code), n (%) | |
| RCA | 24 (26.09) |
| LAD | 63 (68.48) |
| LCx | 5 (5.43) |
| Lesion class, n (%) | |
| A | 11 (11.96) |
| B1 | 41 (44.57) |
| B2 | 17 (18.48) |
| C | 23 (25.00) |
| Stenosis type, n (%) | |
| de novo | 92 (100) |
| In-stent | 0 |
| Bifurcation | 0 |
| Characteristics | Treated lesions (n = 92)/patients (n = 88) |
|---|---|
| SD: standard deviation; TIMI: thrombolysis in myocardial infarction. | |
| Total number of lesions treated with study device | 92 |
| Total number of study device deployed | 92 |
| Study stents per patient | 1.05 |
| Totally occluded | 23 (25.00) |
| Diameter stenosis (%), mean ± SD | 90.17 ± 9.93 |
| Average lesion length, mm, mean ± SD | 43.32 ± 9.46 |
| Procedure access site location, n (%) | |
| Femoral right | 86 (97.73) |
| Radial right | 2 (2.27) |
| Contrast media used, n (%) | |
| Ionic | 60 (68.18) |
| Non-ionic | 28 (31.82) |
| TIMI flow pre-procedure, n (%) | |
| 0 | 21 (22.83) |
| 1 | 28 (30.43) |
| 2 | 43 (46.74) |
| 3 | 0 |
| TIMI flow post-procedure, n (%) | |
| 0 | 0 |
| 1 | 0 |
| 2 | 0 |
| 3 | 92 (100) |
| Pre-dilatation, n (%) | 83 (90.22) |
| Post-dilatation, n (%) | 72 (78.26) |
| Average stent length, mm, mean ± SD | 45.54 ± 10.20 |
| Average stent diameter, mm, mean ± SD | 2.99 ± 0.29 |
| Stent length (mm), n (%) | |
| 30 | 17 (18.48) |
| 40 | 26 (28.26) |
| 50 | 30 (32.61) |
| 60 | 19 (20.65) |
| Stent diameter (mm), n (%) | |
| 3.00 - 2.50 | 34 (36.96) |
| 3.50 - 3.00 | 49 (53.26) |
| 2.75 - 2.25 | 9 (9.78) |
| 3.50 - 2.75 | 0 |
| Procedure success, n (%) | 88 (100) |
| Device success, n (%) | 88 (100) |
| Events, n (%) | In-hospital (n = 88) | Follow-up | ||||
|---|---|---|---|---|---|---|
| 1 month (n = 88) | 6 months (n = 88) | 12 months (n = 88) | 24 months (n = 88) | 36 months (n = 88) | ||
| aSeven patients suffered from MI and cardiac death. ID-TLR: ischemia-driven target lesion revascularization; ID-TVR: ischemia-driven target vessel revascularization; MACE: major adverse cardiovascular event; MI: myocardial infarction; ST: stent thrombosis; TLF: target lesion failure; TVF: target vessel failure. | ||||||
| All-cause death | 0 | 1 (1.14) | 4 (4.55) | 8 (9.09) | 10 (11.36) | 11 (12.50) |
| Cardiac deatha | 0 | 1 (1.14) | 3 (3.41) | 6 (6.82) | 7 (7.95) | 7 (7.95) |
| Non-cardiac death | 0 | 0 | 1 (1.14) | 2 (2.27) | 3 (3.41) | 4 (4.55) |
| MIa | 0 | 1 (1.14) | 3 (3.41) | 6 (6.82) | 7 (7.95) | 7 (7.95) |
| ID-TLR | 0 | 0 | 0 | 0 | 0 | 0 |
| ID-TVR | 0 | 0 | 0 | 0 | 0 | 0 |
| ST | 0 | 0 | 0 | 0 | 0 | 0 |
| TVF | 0 | 1 (1.14) | 3 (3.41) | 6 (6.82) | 7 (7.95) | 7 (7.95) |
| TLF | 0 | 1 (1.14) | 3 (3.41) | 6 (6.82) | 7 (7.95) | 7 (7.95) |
| MACE | 0 | 1 (1.14) | 3 (3.41) | 6 (6.82) | 7 (7.95) | 7 (7.95) |
| Freedom from TLF | 88 (100.0) | 87 (98.86) | 85 (96.59) | 82 (93.18) | 81 (92.05) | 81 (92.05) |
| Variables/stents | BioMime™ Morph (current study) | Kang et al, 2022 [23] | Gautier et al, 2022 [26] | Hsiao et al, 2022 [27] | Karmpaliotis et al, 2022 [28] | Sim et al, 2020 [29] | Paszek et al, 2019 [30] | Diaz Fernnndez et al, 2018 [31] | Rajesh et al, 2018 [32] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoCr-EES | PtCr-EES | Re-ZES | BP-BES | SES | EES | EES | EES | EES | EES + ZES | EES | EES + SES | |||
| aDefinite ST. bDefinite and probable ST. cIschemia driven. dTotal probable stent thrombosis. eMean stent length per lesion. fProbable ST. gDevice-oriented composite endpoint (DOCE) composed of cardiac death, target vessel-related MI and TLR. hMedian follow-up. iValues are given as percentages. BP-BES: biodegradable polymer biolimus-eluting stents; CoCr-EES: cobalt chromium everolimus-eluting stent; DES: drug-eluting stent; MACE: major adverse cardiovascular event; MI: myocardial infarction; PtCr-EES: platinum-chromium everolimus-eluting stent; Re-ZES: Resolute (®) zotarolimus eluting stent; SES: sirolimus-eluting stent; ST: stent thrombosis; TLR: target lesion revascularization; TVR: target vessel revascularization. | ||||||||||||||
| Number of patients | 88 | 224 | 255 | 250 | 245 | 476 | 268 | 213 | 100 | 117 | 290 | 610 | 343 | |
| Average length of stent, mm | 45.54 ± 10.20 | 46.5 ± 16.9 | 44.5 ± 16.8 | 45.9 ± 17.1 | 40.2 ± 13.4 | 45.6 ± 17.1 | 66 ± 22 | 60.1 ± 20.6e | 50.96 ± 7.25 | 48.00 | 55.5 ± 16.8 | 39.83 ± 14.0 | 41.63 ± 2.77 | |
| Polymer type | Biodegradable | Biodegradable/permanent | Biodegradable/permanent | Biodegradable | Biodegradable | Permanent | Permanent | Permanent | Biodegradable | Permanent | Permanent | Permanent | NA | |
| Drug | Sirolimus | Everolimus | Everolimus | Zotarolimus | Biolimus | Sirolimus | Everolimus | Everolimus | Everolimus | Everolimus | Everolimus and zotarolimus | Everolimus | Everolimus and sirolimus | |
| Clinical follow-up | 12-month | 36-month | 12-month | 12-month | 12-month | 12-month | 12-month | 12-month | 12-month | 12-month | 12-month | 831 daysh (range: 390 - 1,373; interquartile range: 459) | 12-month | 12-month (n = 314) |
| Clinical outcomes, n (%) | ||||||||||||||
| Cardiac death | 6 (6.82) | 7 (7.95) | 0 | 1 (0.4) | 1 (0.4) | 2 (0.8) | 2 (0.4) | 2 (0.7) | 3 (1.4) | 1 (1.0) | 1 (0.9) | 21 (6.9) | 1 (0.2) | 5 (1.6) |
| MI | 6 (6.82) | 7 (7.95) | 22 (9.8) | 40 (15.7) | 29 (11.6) | 34 (13.9) | 51 (10.7) | 1.2i | 3 (1.4) | 2 (2.0) | 4 (3.4) | 19 (6.6) | 8 (1.3) | 2 (0.6) |
| ST | 0 | 0 | 1 (0.4) | 0 | 0 | 3 (1.2) | 2 (0.4) | 2 (0.7)a | 2 (0.9) | 0 | 1 (0.9)f | 21 (7.2)b | 4 (0.7) | 7 (2.2)d |
| MACE | 6 (6.82) | 7 (7.95) | 32 (14.3) | 42 (16.5) | 35 (14.0) | 41 (16.7) | 62 (13.0) | NA | NA | NA | 7 (6.0) | 39 (13.4)g | 13 (2.1) | 19 (6) |
| TLR | 0c | 0c | 7 (3.1) | 5 (2.0) | 4 (1.6) | 8 (3.3) | 9 (1.9) | 4.1i | NA | 1 (1.0) | 1 (0.9) | NA | 4 (0.7) | 3 (1) |
| TVR | 0c | 0c | 9 (4.0) | 5 (2.0) | 5 (2.0) | 9 (3.7) | 10 (2.1) | NA | 7 (3.3) | 1 (1.0) | 1 (0.9) | 18 (6.2) | NA | NA |