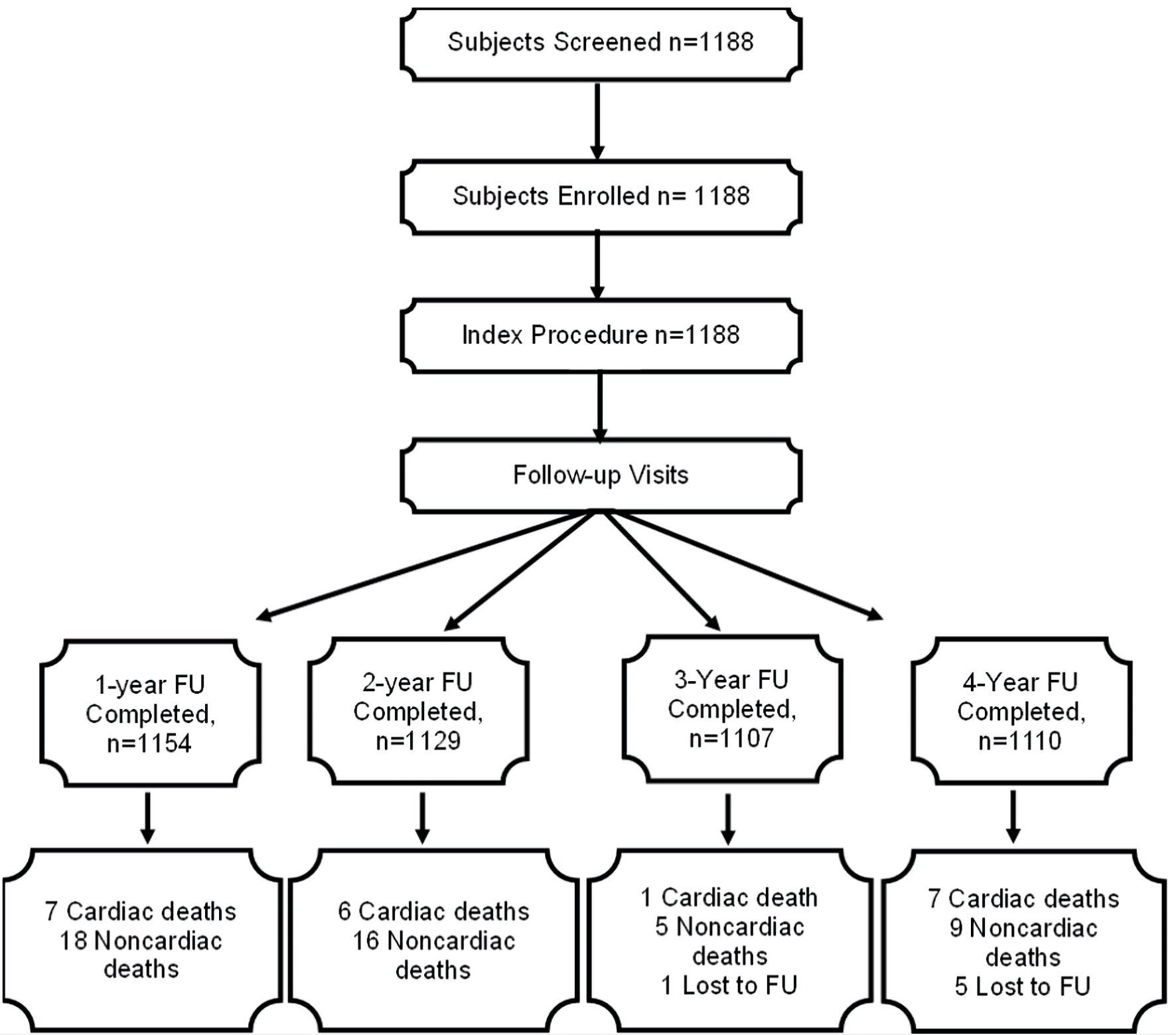
Figure 1. The STROBE flow diagram of patients (non-cumulative) who were enrolled and followed-up for 4 years. STROBE: Strengthening the Reporting of Observational Studies in Epidemiology; FU: follow-up.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 5, October 2023, pages 360-369
Long-Term Assessment of Thin-Strut BioMime Coronary Stent System in Real-World Population at Single-Center: A Retrospective Observational Study
Figure

Tables
| Characteristics | Patients (n = 1,188) |
|---|---|
| N (%) or mean ± SD | |
| Values are expressed as n (%) or mean ± SD. ACS: acute coronary syndrome; bpm: beats per minute; CABG: coronary artery bypass grafting; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-elevation MI; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; SD: standard deviation; STEMI: ST-elevation MI. | |
| Patient demographics | |
| Age (years) | 53.26 ± 10.31 |
| Male | 1,024 (86.20) |
| Female | 164 (13.80) |
| Heart rate, bpm | 80.60 ± 7.19 |
| Systolic blood pressure, mm Hg | 120.34 ± 15.48 |
| Diastolic blood pressure, mm Hg | 76.54 ± 7.87 |
| Serum creatinine, mg/dL | 1.03 ± 1.28 |
| Medical history | |
| Hypertension | 273 (22.98) |
| Diabetes mellitus | 202 (17.00) |
| Hypercholesterolemia | 3 (0.25) |
| Ischemic heart disease | 2 (0.17) |
| Thrombolysis | 19 (1.60) |
| Current smokers | 650 (54.71) |
| Alcohol consumption | 123 (10.35) |
| Stroke | 4 (0.34) |
| COPD | 4 (0.34) |
| Previous CAD | 19 (1.60) |
| Stable angina | 1,007 (84.76) |
| ACS | 181 (15.15) |
| Unstable angina | 37 (3.11) |
| STEMI | 134 (11.28) |
| NSTEMI | 10 (0.84) |
| Other illnesses | 13 (1.09) |
| History of previous interventions | |
| Previous CABG | 1 (0.08) |
| Previous PCI | 28 (2.36) |
| Primary PTCA | 75 (6.31) |
| LVEF, % | 47.38 ± 10.08 |
| Variable | N (%) or mean ± SD |
|---|---|
| Values are expressed as n (%) or mean ± SD. SD: standard deviation. | |
| Target coronary vessel | |
| Left anterior descending | 514 (40.89) |
| Right coronary artery | 500 (39.78) |
| Left circumflex | 232 (18.46) |
| Others | 11 (0.87) |
| Lesion location | |
| Ostial | 8 (0.60) |
| Proximal | 488 (36.61) |
| Mid | 594 (44.56) |
| Distal | 243 (18.23) |
| Lesion characteristics | |
| Bifurcation | 106 (7.9) |
| Chronic total occlusion | 327 (25.31) |
| Vessel disease | |
| Single vessel disease | 675 (56.82) |
| Double vessel disease | 130 (10.94) |
| Multiple vessel disease | 383 (32.24) |
| Lesion details | |
| Total number of lesions | 1,565 |
| Total number of lesions treated | 1,333 |
| Lesion per patient | 1.12 |
| Mean lesion diameter (mm) | 3.01 ± 0.29 |
| Mean lesion length (mm) | 29.62 ± 9.62 |
| Stent details | |
| Total number of study stents deployed | 1,344 |
| Stents per patient | 1.13 |
| Mean diameter of study device (mm) | 3.17 ± 0.25 |
| Mean length of study device (mm) | 30.89 ± 6.31 |
| Total number of other stents deployed | 240 |
| Total number of lesions treated with other stents | 232 |
| Mean stent length (mm) | 26.83 ± 9.23 |
| Mean stent diameter (mm) | 2.89 ± 0.61 |
| P2Y12 inhibitor therapy | |
| Clopidogrel | 725 (61.03) |
| Prasugrel | 154 (12.96) |
| Ticagrelor | 309 (26.01) |
| Event | 1-year | 2-year | 3-year | 4-year |
|---|---|---|---|---|
| N = 1,154 | N = 1,154 | N = 1,153a | N = 1,148a | |
| aLost to follow-up. Values are expressed as n (%). MACE is defined as a composite of cardiac death, myocardial infarction (MI) attributed to target vessel and target lesion revascularization. ST: stent thrombosis; TLR: target lesion revascularization; TVR: target vessel revascularization; MI: myocardial infarction; TVMI: target vessel myocardial infarction. | ||||
| All-death | 25 (2.17) | 47 (4.07) | 53 (4.60) | 69 (6.01) |
| Cardiac | 7 (0.61) | 13 (1.13) | 14 (1.22) | 21 (1.83) |
| Non-cardiac | 18 (1.56) | 34 (2.95) | 39 (3.38) | 48 (4.18) |
| MI | ||||
| TVMI | 0 | 0 | 0 | 0 |
| Non-TVMI | 1 (0.09) | 1 (0.09) | 1 (0.09) | 1 (0.09) |
| TLR | 0 | 4 (0.35) | 10 (0.87) | 18 (1.57) |
| TVR | 0 | 7 (0.61) | 17 (1.47) | 27 (2.35) |
| ST | 3 (0.3) | 3 (0.3) | 3 (0.3) | 3 (0.3) |
| MACE | 7 (0.61) | 17 (1.47) | 24 (2.08) | 39 (3.40) |
| BioMime stent length | 1-year | 2-year | 3-year | 4-year | ||||
|---|---|---|---|---|---|---|---|---|
| ≥ 35 mm (n = 393) | < 35 mm (n = 761) | ≥ 35 mm (n = 393) | < 35 mm (n = 761) | ≥ 35 mm (n = 393) | < 35 mma (n = 760) | ≥ 35 mm (n = 393) | < 35 mma (n = 755) | |
| aLost to follow-up. Values are expressed as n (%). MACE is defined as a composite of cardiac death, myocardial infarction (MI) attributed to target vessel and target lesion revascularization. ST: stent thrombosis; TLR: target lesion revascularization; TVR: target vessel revascularization; MI: myocardial infarction; TVMI: target vessel myocardial infarction. | ||||||||
| All-death | 12 (3.0) | 13 (1.7) | 21 (5.3) | 26 (3.4) | 20 (5.1) | 33 (4.3) | 26 (6.6) | 43 (5.7) |
| Cardiac | 3 (7.6) | 4 (0.5) | 5 (1.3) | 8 (1.0) | 5 (1.3) | 9 (1.2) | 7 (1.8) | 14 (1.9) |
| Non-cardiac | 9 (2.3) | 9 (1.2) | 16 (4.1) | 18 (2.4) | 15 (3.8) | 24 (3.1) | 19 (4.8) | 29 (3.8) |
| MI | ||||||||
| TVMI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-TVMI | 0 | 1 (0.1) | 0 | 1 (0.1) | 0 | 1 (0.1) | 0 | 1 (0.1) |
| TLR | 0 | 0 | 2 (0.5) | 2 (0.3) | 2 (0.5) | 8 (1.0) | 2 (0.5) | 16 (2.1) |
| TVR | 0 | 0 | 4 (1.0) | 3 (0.4) | 4 (1.0) | 13 (1.7) | 4 (1.0) | 23 (3.0) |
| ST | 1 (0.3) | 2 (0.3) | 1 (0.3) | 2 (0.3) | 1 (0.3) | 2 (0.3) | 1 (0.3) | 2 (0.3) |
| MACE | 3 (0.8) | 4 (0.5) | 8 (2.0) | 9 (1.2) | 8 (2.0) | 17 (2.2) | 10 (2.5) | 23 (3.0) |
| Study name | Study design | Device name | Strut thickness | Sample size (FU number/enrolled number) | Follow-up duration | Cardiac death | MI | TLR/CD-TLR/CI-TLR | TVR | ST | MACE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD-TLR: clinically-driven target lesion revascularisation; CI-TLR: clinically-indicated target lesion revascularisation; DES: drug-eluting stent; FU: follow-up; MI: myocardial infarction; MACE: major adverse cardiac event; POCO: patient-oriented composite outcomes; RES: rapamycin-eluting stent; SES: sirolimus-eluting stent; ST: stent thrombosis; TLF: target lesion failure; TLR: target lesion revascularisation; TVR: target vessel revascularization; TVMI: target vessel myocardial infarction. | |||||||||||
| BioMime GBPR (current study) | Retrospective, real-world registry | BioMime SES | 65 µm | 1,148/1,188 | 4 years | 1.83% | TVMI: 0%, non-TVMI: 0.09% | 1.57% | 2.35% | 0.3% | 3.4% |
| 1,154/1,188 | 1 year | 0.61% | TVMI: 0%, non-TVMI: 0.09% | 0% | 0% | 0.3% | 0.61% | ||||
| FLEX registry [20] | Retrospective, observational registry | Suprafelx SES | 60 µm | 980/1,995 | 1 year | 1.8% | 1.6% | 0.7% | Non-TL TVR: 0.2% | 1.1% | 3.7% |
| Tetriflex SES [21] | Retrospective, observational registry | Tetriflex SES | 60 µm | 1,218/1,269 | 1 year | 0.82% | TVMI: 3.20% | 1.72% | - | 0.65% | TLF: 5.75% |
| T-FLEX registry [22] | Retrospective, observational registry | Biodegradable polymer coated SES | 60 µm | 1,143/1,203 | 1 year | 0.6% | 2.1% | 1.9% | - | 0.8% | TLF: 3.8% |
| Thailand Orsiro registry [23] | Prospective, observational registry | Orsiro SES | 60 µm | 139/150 | 1 year | 5.3% | 1.3% | 0% | 0.7% | 1.3% | TLF: 5.3% |
| FlexyRap® DES study [24] | Retrospective, observational, post-marketing study | FlexyRap RES | 60 µm | 500 | 5 years | 0% | TVMI: 0.4% | 0% | 0% | Late ST: 0.4%, very late ST: 0.4% | 0.4% |
| Genoss DES™ prospective registry [25] | Prospective, single-arm observational, multicenter registry | Genoss DES™ SES | 70 µm | 622 | 1 year | 0.2% | Any MI: 0.6% | 0.5% | 0.8% | 0.6% | POCO: 3.9% (including any death, any MI, and any revascularization) |
| BIOFLOW-VII [26] | Prospective, multicenter single-arm US post-marketing study | Orsiro SES | 60 µm | 556 | 1 year | 0% | Any MI: 1.7% | 0.9% | 2.3% | 0.4% | 3.2% |
| The e-Cobra study [27] | Prospective, multicenter, observational study | Cobra PzF Polyzene F nano coating stent | 70 µm | 915/940 | 1 year | 3.7% | 4.8% | 4.3% | 5.0% | Definite: 0.7%, probable: 0.7%, possible: 2.3% | 8.6% (cardiac death 3.7%, or MI 4.8%, or TLR: 4.3%) |
| LEADERS FREE III study [28] | Prospective, multicenter, single-arm study | BioFreedom™ Biolimus-A9-eluting Co-Cr stent | 84 - 88 µm | 401 | 1 year | 3.7% | 4.4% | 4.2% | 5.0% | 1.0% | - |