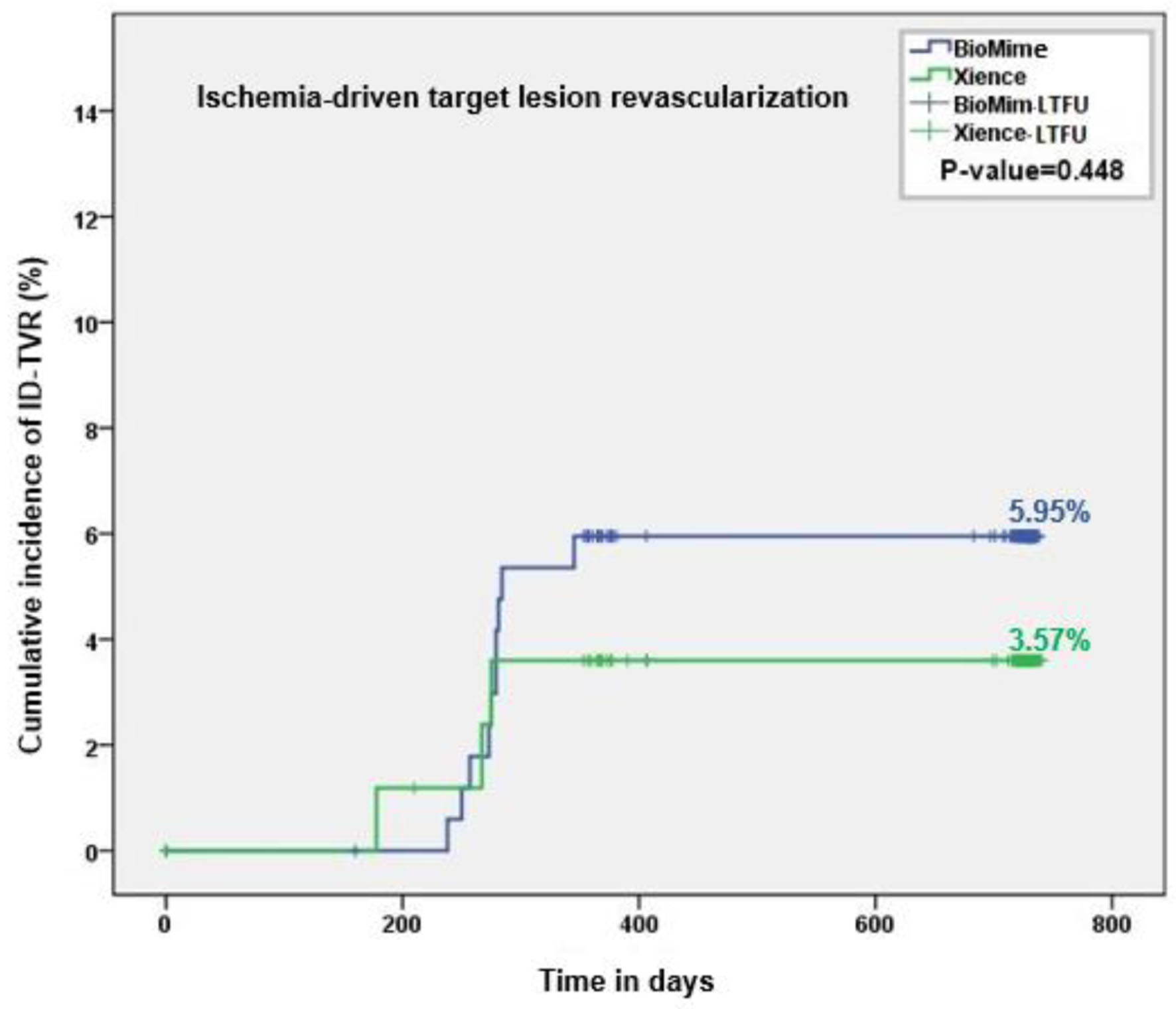
Figure 1. Kaplan-Meier analysis of ID-TVR up to the 2-year follow-up. ID-TVR: ischemia-driven target vessel revascularization.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 14, Number 4, August 2023, pages 291-301
A Randomized Controlled Trial Comparing BioMime Sirolimus-Eluting Stent With Everolimus-Eluting Stent: Two-Year Outcomes of the meriT-V Trial
Figures

Tables
| Stent | Alloy | Strut thickness | Drug (dose density) | Polymer | Coating thickness | Bare metal platform | Biodegradability of polymer coating (yes/no) | Polymer degradation (months) |
|---|---|---|---|---|---|---|---|---|
| DES: drug-eluting stent; PLLA: poly-L-lactic acid; PLGA: poly-lactic co-glycolic acid; PVDF: polyvinylidine fluoride. | ||||||||
| XIENCE V (Abbott Vascular) | L605 CoCr | 81 µm | Everolimus (1.0 µg/mm2) | Permanent fluorinated PVDF polymer | 7.8 | Vision® | - | Not applicable |
| BioMime (Meril Life Science) | L605 CoCr | 65 µm | Sirolimus (1.25 µg/mm2) | PLLA-PLGA | 2 | Nextgen™ (Meril Life Sciences Pvt. Ltd., India) | ✓ | 9 |
| meriT-1 [9] | meriT-2 [13] | meriT-3 [14] | meriT-V [15] |
|---|---|---|---|
| CAD: coronary artery disease; ID-TLR: Ischemia-driven target lesion revascularization; ID-TVR: Ischemia-driven target vessel revascularization; IHD: ischemic heart disease; LLL: late lumen loss; MACE: major adverse cardiac event; ST: stent thrombosis; TV-MI: target vessel-related myocardial infarction. | |||
| Single-center/first in man | Multicenter | Multicenter/post-marketing | Multicenter |
| India | India (11 sites) | India (15 sites) | Europe (15 sites), Brazil (three sites) |
| 30 patients/30 de novo lesions | 250 patients/355 de novo lesions | All-comers patients 1,161/1,312 lesions | Randomized; 256 patients (BioMime 170/182 lesions vs. 86/95 lesions XIENCE) |
| 8-month angiographic follow-up | 8-month angiographic follow-up | - | 9-month angiographic follow-up |
| Key results (1 year) | Key results (1 year) | Key results (1 year) | Key results (9-month follow-up) |
| Procedural success: 100% | Procedural success: 99.2% | High procedural success rate | Procedural success: BioMime 99.41% vs. 98.84% |
| In-stent LLL: 0.15 mm; in segment: 0.17 mm; binary restenosis: 0% | In-stent LLL: 0.12 mm; in segment: 0.11 mm | - | - |
| MACE (cardiac death, MI and TLR); ST: 0% | MACE (cardiac death 0.8%, MI 0.4% or any TLR 5.2%): 6%; ST: 0.4% | MACE (cardiac death 1.4%, MI 0.35% and any TLR 0.52%): 2.35%; ST: 0.1% | MACE (cardiac death, any MI and ID-TVR): BioMime 2.98 vs. XIENCE 7.14%; BioMime vs. XIENCE; Death 0% for both; MI 0.6% vs. 4.76% (any MI); ID-TLR/ID-TVR: 2.38% in both groups; ST: 0% in both groups; TV-MI: BioMime 0.6% vs. XIENCE 1.19% |
| Indications: silent ischemia | CAD/IHD | CAD | IHD |
| Variables | BioMime™ SES (N = 170) | XIENCE EES (N = 86) | P-value (BioMime™ SES vs. XIENCE EES) |
|---|---|---|---|
| CAD: coronary artery disease; LVEF: left ventricular ejection fraction; MI: myocardial infarction; N: number of patients; PCI: percutaneous coronary intervention; SD: standard deviation; ST: stent thrombosis; STEMI: ST elevation myocardial infarction. | |||
| Age (years), mean ± SD | 64.33 ± 9.57 | 64.70 ± 8.99 | 0.75 |
| Male, n (%) | 111 (65.29) | 53 (61.63) | 0.56 |
| Body mass index (kg/m2) | 28.64 ± 4.45 | 29.40 ± 4.39 | 0.20 |
| Cardiac risk factors, n (%) | |||
| Diabetes mellitus | 41 (24.12) | 18 (20.93) | 0.57 |
| Hypertension | 125 (73.53) | 68 (79.07) | 0.11 |
| Dyslipidemia | 118 (69.41) | 59 (68.60) | 0.89 |
| Chronic lung disease | 9 (5.29) | 10 (11.63) | 0.07 |
| Smokers | 71 (41.76) | 41 (47.67) | 0.37 |
| History of CAD | 67 (39.41) | 29 (33.72) | 0.37 |
| Renal insufficiency | 4 (2.35) | 2 (2.33) | 0.99 |
| Previous MI | 37 (21.76) | 13 (15.12) | 0.25 |
| Previous PCI | 31 (18.24) | 14 (16.28) | 0.69 |
| Cardiac status, n (%) | |||
| Stable angina | 116 (68.24) | 61 (70.9) | 0.66 |
| Unstable angina | 25 (14.71) | 12 (13.95) | 0.87 |
| Asymptomatic | 16 (9.41) | 5 (5.81) | 0.32 |
| STEMI | 3 (1.76) | 0 (0.0) | 0.25 |
| NSTEMI | 10 (5.88) | 8 (9.3) | 0.31 |
| LVEF, % | 55.86 ± 7.22 | 56.82 ± 10.13 | 0.40 |
| Clinical events | Follow-up | ||||||
|---|---|---|---|---|---|---|---|
| In-hospital (N = 256) | 1 year (N = 252) | 2 years (N = 252) | P-value (at 2 years) | ||||
| BioMime (N = 170) | XIENCE (N = 86) | BioMime (N = 168) | XIENCE (N = 84) | BioMime (N = 168) | XIENCE (N = 84) | BioMime vs. XIENCE | |
| Values are presented as n (%). ID-TLR: ischemia-driven target lesion revascularization; ID-TVR: ischemia-driven target vessel revascularization; MACE: major adverse cardiac event; N: number of patients. | |||||||
| Death, n (%) | |||||||
| Cardiac death | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Non-cardiac death | 0 | 0 | 1 (0.6%) | 0 | 2 (1.19%) | 0 | 0.32 |
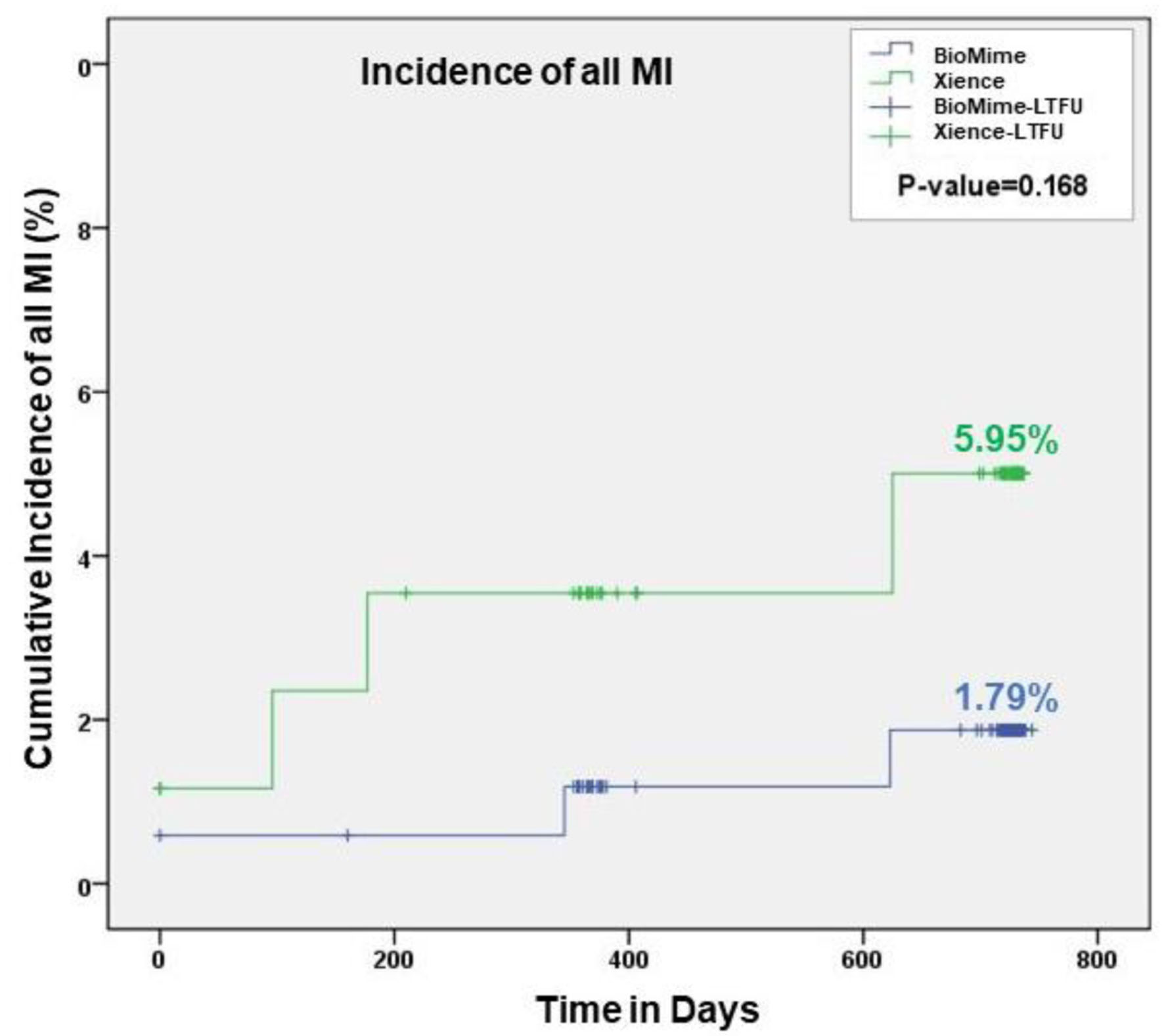
| Myocardial infarction | 1 (0.59%) | 1 (1.16%) | 2 (1.19 %) | 4 (4.76%) | 3 (1.79%) | 5 (5.95%) | 0.17 |
| ID-TLR | 0 | 0 | 10 (5.95%) | 3 (3.57%) | 10 (5.95%) | 3 (3.57%) | 0.45 |
| ID-TVR (including TLR) | 0 | 0 | 10 (5.95%) | 3 (3.57%) | 10 (5.95%) | 3 (3.57%) | 0.45 |
| ID-TVR (non-TLR) | 0 | 0 | 4 (2.38%) | 2 (2.38%) | 4 (2.38%) | 2 (2.38%) | 0.99 |
| Stent thrombosis, n (%) | |||||||
| Definite or probable/probable | 0 | 0 | 0 | 1 (1.19%) | 0 | 1 (1.19%) | 0.16 |
| Total of three-point MACE | 1 (0.59%) | 1 (1.16%) | 11 (6.55%) | 7 (8.33%) | 13 (7.74%) | 8 (9.52%) | 0.6170 |
| Trials | Study details | Cardiac death | TLR | TVR | MI | TV-MI | Late ST | ST | Other events |
|---|---|---|---|---|---|---|---|---|---|
| Values are n (%). ACS: acute coronary syndrome; BP: biodegradable polymer; CI-TLR: clinically-indicated target lesion revascularization; CI-TVR: clinically-indicated target vessel revascularization; DP: durable polymer; ID-TLR: ischemia-driven target lesion revascularization; ID-TVR: ischemia-driven target vessel revascularization; MACE: major adverse cardiac event; N: number of patients; n-DES: new-generation drug-eluting stent; PCI: percutaneous coronary intervention; ST: stent thrombosis; STEMI: ST elevation myocardial infarction; TLF: target lesion failure; TVF: target vessel failure; TV-MI: target vessel-related myocardial infarction. | |||||||||
| BIOSCIENCE (2-year follow-up) [24] | Orsiro arm: 1,063 patients (1,594 lesions); XIENCE arm: 1,056 patients (1,545 lesions); patients with presence or absence of STEMI | 3.2% vs. 3.2% | 6.4% vs. 5.8 % | 8.1% vs. 7.5% | 6.1% vs. 7.2% | 4.1% vs. 4.5% | - | Definite/probable 3.9% vs. 4.9%; definite 1.1% vs. 0.8% | CI-TVR 7.7% vs. 6.8%; CI-TLR: 6.0% vs. 5.1%; TLF 10.5% vs. 10.4%; TVF 12.2% vs. 12.3% |
| DESSOLVE III (2-year follow-up) [25] | MiSTENT (703/1,398 patients) vs. XIENCE (695/1,398 patients); 20 centers in Europe; all comers | 3.0% vs. 2.0% | 5.1% vs. 6% | 6.9% vs. 8.5% | 3.0% vs. 2.8% | 2.7% vs. 2.3% | Late 0.4% vs. 0.6%; very late ST 0.3% vs. 0.3% | Definite/probable 0.9% vs. 1.3%; definite 0.6% vs. 1% | CI-TVR 5.9% vs. 7.7%; CI-TLR: 4.6% vs. 5.4%; DOCE/TLF: 8.7% vs. 8.6% |
| BIOSTEMI trial (2-year follow-up) [22] | Orsiro (649/1,300 patients/651 lesions) vs. XIENCE (651/1,300 patients/806 lesions); 10 centers in Switzerland; first STEMI | 2.9% vs. 3.2% | 2.8% vs. 5.2% | 3.4% vs. 6.3% | 3.7% vs. 3.1% | 1.5% vs. 2.0% | - | Definite/probable 2.0% vs. 2.3%; definite 1.4% vs. 1.8% | TLF: 5.1% vs. 8.1%; CI-TVR 3.1% vs. 6.1%; CI-TLR: 2.5% vs. 5.1%; TVF 6% vs. 9.4% |
| BIOFLOW-V trial (3-year follow-up) [23] | Orsiro (884/1,334 patients/1,051 lesions) vs. XIENCE (450/1,334 patients/561 lesions); 92 international randomized study centers; non-STEMI/ACS | 1.1% vs. 1.2% | - | - | - | 5% vs. 9.2% | Late and very late ST 0.1% vs. 1.2% | - | ID-TLR: 3.2% vs. 6.7%; MACE: 11.5% vs. 17.5%; TVF: 9.7% vs. 16.2; TLF: 8.2% vs. 13.6% |
| BIOFLOW-II trial (5-year follow-up) [26] | Orsiro (298 patients/332 lesions) vs. XIENCE (154 patients/173 lesions); 24 centers in eight European countries; de novo lesions | 1.7% vs. 2.8% | - | - | 4.5% vs. 6.2% | 3.4% vs. 3.3% | - | Overall ST 0.7% vs. 2.8%; definite 0% vs. 0.7%; probable 0% | CI-TVR 12.6% vs. 10.1%; CI-TLR: 6.3% vs. 6.7%; TLF 10.4% vs. 12.7%; TVF 15.6% vs. 12.7% |
| BIOFLOW-V trial (5-year follow-up) [23] | Orsiro (884 patients) vs. XIENCE (450 patients); 92 randomized study centers; non-STEMI/ACS | 2.6% vs. 1.9% | - | - | 6.6% vs. 10.3% | Late and very late ST 0.3% vs. 1.6% | - | ID-TLR: 5.9% vs. 7.7%; ID-TVR: 9.6% vs. 12.4%; TLF 12.3% vs. 15.3%; TVF 15.2% vs .19%; MACE: 18.3% vs. 21.4% | |
| BIORESORT trial (3-year follow-up) [27] | 1,506/3,514 patients; Orsiro (525) vs. synergy (496) vs. resolute integrity (485); four clinical sites; all-comer trial | 1.3% vs. 1.8% vs. 2.1% | 1.7% vs. 2.5% vs. 4.4% | - | - | 2.7% vs. 2.7% vs. 4.0% | - | Definite or probable ST 0.6% vs. 1.2% vs. 1.5%; definite ST 0.4% vs. 0.6% vs. 1.1% | TLF: 5.2% vs. 6.5% vs. 8.7% |
| SCAAR registry [28] | 4,561 patients implanted with Orsiro and 69,570 n-DES group (XIENCE PRIME, XIENCE Xpedition and XIENCE ProX, PROMUS Element, Plus and Promus PRIMER, Resolute Integrity and Resolute, Onyx, SYNERGY; undergoing PCI | - | 1.6% vs. 2.3% | 6.0% vs. 5.2% | Late ST: 0.5% vs. 0.6%; very late ST: 0.7% vs. 0.8% | Definite ST: 0.7% vs. 0.8% | |||