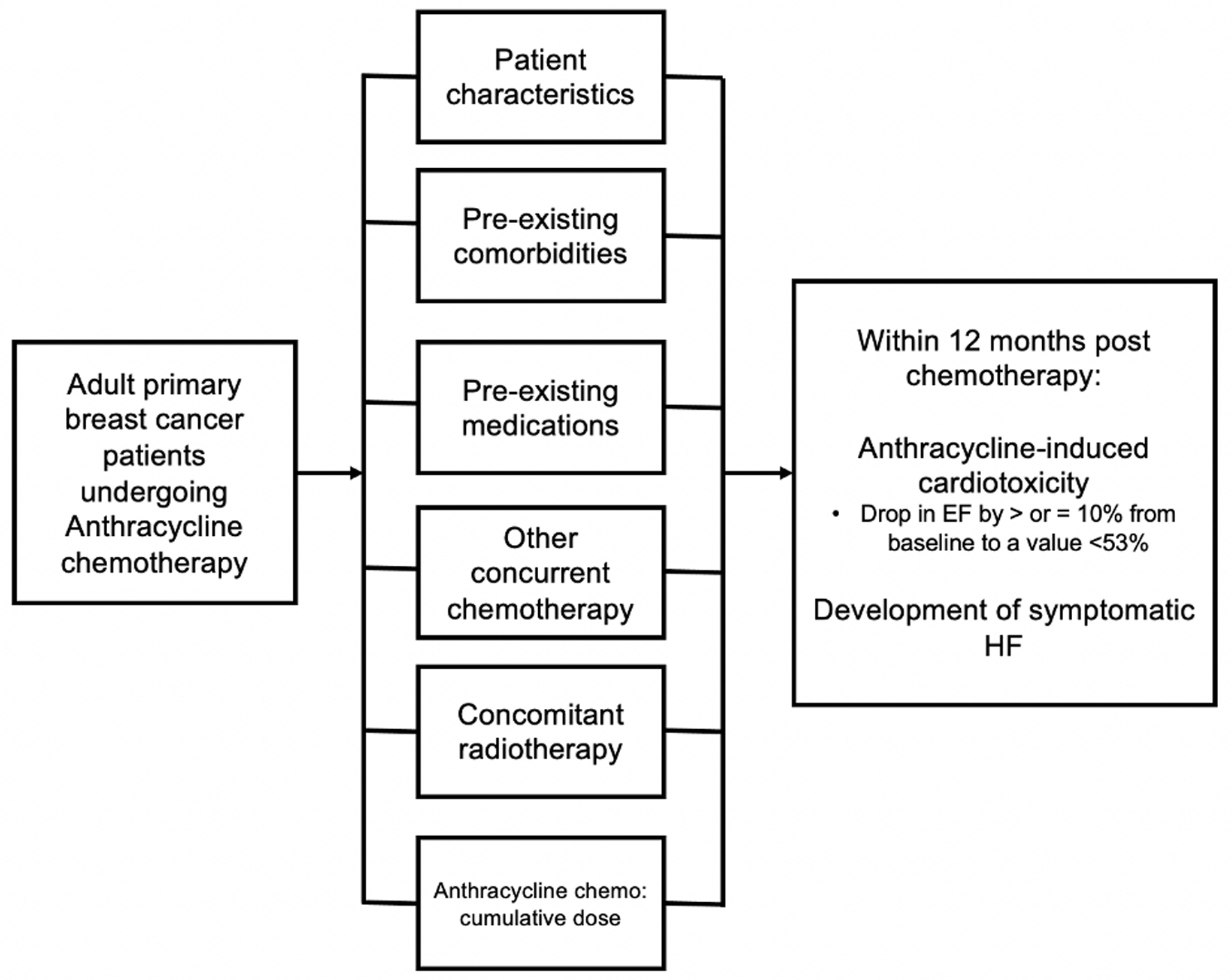
Figure 1. Conceptual framework for this study.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 6, December 2022, pages 380-392
Anthracycline-Induced Cardiotoxicity in Breast Cancer Patients: A Five-Year Retrospective Study in 10 Centers
Figures

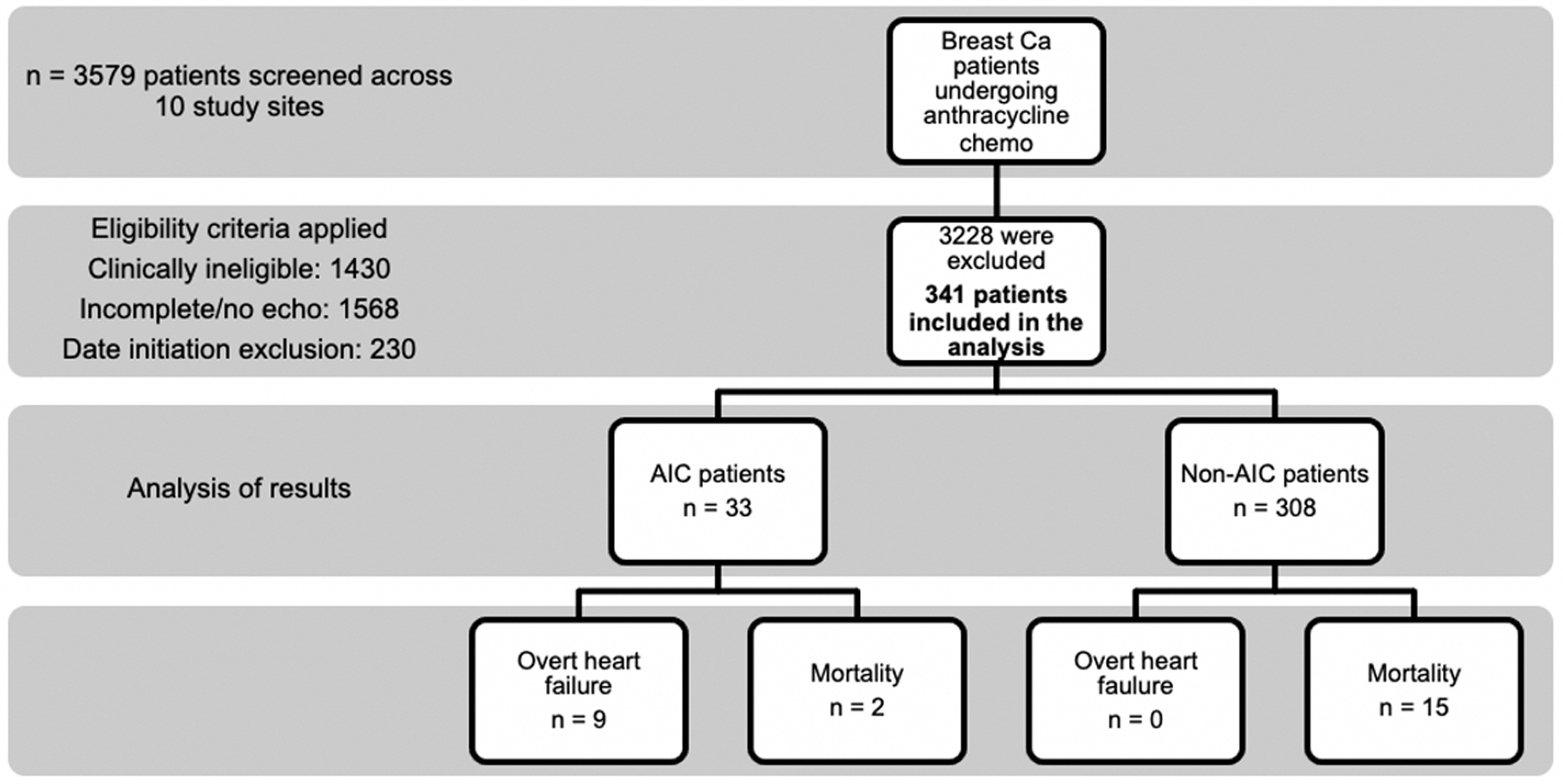
Tables
| Total (n = 341) | With AIC (n = 33) | Without AIC (n = 308) | P-value | |
|---|---|---|---|---|
| Statistical test used: *Independent sample t-test; †Mann-Whitney U test; ‡Chi-square/Fisher’s exact test. Data are expressed frequency (%) or median (range). AIC: anthracycline-induced cardiotoxicity; ARBs: angiotensin receptor blockers; BMI: body mass index; PTB: pulmonary tuberculosis; ACE: angiotensin converting enzyme; SGLT2: sodium-glucose cotransporter-2; HER2: human epidermal growth factor 2; LVEF: left ventricular ejection fraction. | ||||
| Age, years | 53.91 ± 10.84 | 59.06 ± 9.7 | 53.36 ± 10.83 | 0.004* |
| BMI, kg/m2 | 25.3 (11.3 - 45.06) | 24 (11.7 - 34.71) | 25.3 (11.3 - 45.06) | 0.003† |
| BMI category | ||||
| Underweight (< 18.5) | 12 (3.52) | 2 (6.06) | 10 (3.25) | 0.336‡ |
| Normal (18.5 to < 23) | 84 (24.63) | 10 (30.3) | 74 (24.03) | |
| Overweight (23 to 27.5) | 134 (39.3) | 9 (27.27) | 125 (40.58) | |
| Obese (> 27.5) | 111 (32.55) | 12 (36.36) | 99 (32.14) | |
| Comorbidities | ||||
| Any | 154 (45.16) | 27 (81.82) | 127 (41.23) | < 0.001‡ |
| Hypertension | 114 (33.43) | 22 (66.67) | 92 (29.87) | < 0.001‡ |
| Diabetes mellitus | 57 (16.72) | 7 (21.21) | 50 (16.23) | 0.464‡ |
| Dyslipidemia | 36 (10.56) | 3 (9.09) | 33 (10.71) | > 0.999‡ |
| PTB | 6 (1.76) | 1 (3.03) | 5 (1.62) | 0.460‡ |
| Asthma | 4 (1.17) | 0 | 4 (1.3) | > 0.999‡ |
| Atrial fibrillation | 3 (0.88) | 2 (6.06) | 1 (0.32) | 0.026‡ |
| Coronary artery disease | 3 (0.88) | 1 (3.03) | 2 (0.65) | 0.264‡ |
| Chronic kidney disease | 3 (0.88) | 1 (3.03) | 2 (0.65) | 0.264‡ |
| Hyperthyroidism | 3 (0.88) | 1 (3.03) | 2 (0.65) | 0.264‡ |
| Hepatitis B | 2 (0.59) | 0 | 2 (0.65) | > 0.999‡ |
| Other | 23 (6.74) | 3 (9.09) | 20 (6.49) | 0.477‡ |
| Medications | ||||
| Any medications | 130 (38.12) | 20 (60.61) | 110 (35.71) | 0.008‡ |
| Calcium channel blocker | 55 (16.13) | 8 (24.24) | 47 (15.26) | 0.211‡ |
| ARBs | 53 (15.54) | 13 (39.39) | 40 (12.99) | < 0.001‡ |
| Statin | 39 (11.44) | 5 (15.15) | 34 (11.04) | 0.562‡ |
| Oral hypoglycemic agent | 37 (10.85) | 4 (12.12) | 33 (10.71) | 0.769‡ |
| Beta-blocker | 19 (5.57) | 6 (18.18) | 13 (4.22) | 0.006‡ |
| ACE inhibitor | 7 (2.05) | 3 (9.09) | 4 (1.3) | 0.022‡ |
| Anti-hypertensive | 5 (1.47) | 2 (6.06) | 3 (0.97) | 0.076‡ |
| Anti-platelet | 5 (1.47) | 1 (3.03) | 4 (1.3) | 0.401‡ |
| Exogenous insulin | 5 (1.47) | 0 | 5 (1.62) | > 0.999‡ |
| Exogenous thyroid medication | 3 (0.88) | 0 | 3 (0.97) | > 0.999‡ |
| Anticholesterol medication | 3 (0.88) | 0 | 3 (0.97) | > 0.999‡ |
| Vitamins | 3 (0.88) | 0 | 3 (0.97) | > 0.999‡ |
| Cardiometabolic drugs | 3 (0.88) | 1 (3.03) | 2 (0.65) | 0.264‡ |
| Anti-arrhythmic medication | 2 (0.59) | 1 (3.03) | 1 (0.32) | 0.184‡ |
| Anti-thyroid medication | 2 (0.59) | 0 | 2 (0.65) | > 0.999‡ |
| Novel oral anticoagulant | 2 (0.59) | 1 (3.03) | 1 (0.32) | 0.184‡ |
| Nutritional supplements | 2 (0.59) | 1 (3.03) | 1 (0.32) | 0.184‡ |
| SGLT2 inhibitors | 2 (0.59) | 1 (3.03) | 1 (0.32) | 0.184‡ |
| Anti-asthma medications | 1 (0.29) | 0 | 1 (0.32) | > 0.999‡ |
| Oral antimetabolite | 1 (0.29) | 0 | 1 (0.32) | > 0.999‡ |
| Anti-tuberculosis medications | 1 (0.29) | 0 | 1 (0.32) | > 0.999‡ |
| Low molecular weight heparin | 1 (0.29) | 0 | 1 (0.32) | > 0.999‡ |
| Mineralocorticoid receptor antagonists | 0 | 0 | 0 | - |
| Concurrent chemotherapeutic agents | ||||
| Any | 319 (93.55) | 21 (63.64) | 298 (96.75) | < 0.001‡ |
| Cyclophosphamide | 262 (76.83) | 12 (36.36) | 250 (81.17) | < 0.001‡ |
| Taxanes | 230 (67.45) | 13 (39.39) | 217 (70.45) | 0.001‡ |
| HER-2 inhibitors | 119 (34.9) | 7 (21.21) | 112 (36.36) | 0.088‡ |
| 5-fluorouracil | 54 (15.84) | 1 (3.03) | 53 (17.21) | 0.041‡ |
| Carboplatin | 14 (4.11) | 0 | 14 (4.55) | 0.377‡ |
| Monoclonal antibodies | 2 (0.59) | 0 | 2 (0.65) | > 0.999‡ |
| Eribulin | 2 (0.59) | 1 (3.03) | 1 (0.32) | 0.184‡ |
| Vinorelbine | 1 (0.29) | 1 (3.03) | 0 | 0.097‡ |
| Tyrosine kinase inhibitors | 0 | 0 | 0 | - |
| Radiation therapy | > 0.999‡ | |||
| No radiotherapy | 303 (88.86) | 30 (90.91) | 273 (88.64) | |
| Mediastinal radiotherapy | 7 (2.05) | 0 | 7 (2.27) | |
| Left breast radiotherapy | 19 (5.57) | 2 (6.06) | 17 (5.52) | |
| Right chest wall radiotherapy | 12 (3.52) | 1 (3.03) | 11 (3.57) | |
| Baseline LVEF | 67.53 ± 5.01 | 65.89 ± 5.84 | 67.71 ± 4.89 | 0.047* |
| Total (n = 341) | With AIC (n = 33) | Without AIC (n = 308) | P-value | |
|---|---|---|---|---|
| Statistical test used: *Independent sample t-test; †Mann-Whitney U test. Data are expressed frequency (%) or median (range). AIC: anthracycline-induced cardiotoxicity. | ||||
| Location of initiation chemotherapy | 0.147‡ | |||
| In-patient/admitted | 155 (45.45) | 19 (57.58) | 136 (44.16) | |
| Outpatient | 186 (54.55) | 14 (42.42) | 172 (55.84) | |
| Dosage | ||||
| 5-fluorouracil, mg/m2 | 316.69 ± 1,081.76 | 92.03 ± 522.07 | 340.76 ± 1,123.2 | 0.210* |
| Epirubicin, mg/m2 | 65.13 ± 141.9 | 14.36 ± 45.24 | 70.57 ± 147.58 | 0.030* |
| Doxorubicin, mg/m2 | 96.82 ± 126.53 | 132.36 ± 127.24 | 93.02 ± 126.06 | 0.090* |
| Idarubicin, mg/m2 | 4.69 ± 63.07 | 0 | 5.19 ± 66.35 | 0.654* |
| Daunorubicin, mg/m2 | 0 | 0 | 0 | - |
| Trastuzumab, mg | 143.77 ± 627.86 | 57.03 ± 115.75 | 153.07 ± 659.01 | 0.405* |
| Docetaxel, mg/m2 | 96.98 ± 141.01 | 82.88 ± 154.53 | 98.49 ± 139.68 | 0.546* |
| Paclitaxel, mg/m2 | 69.77 ± 221.34 | 50.62 ± 171.5 | 71.83 ± 226.16 | 0.602* |
| Cyclophosphamide, mg | 1,352.6 ± 1,247.64 | 1,447.39 ± 1,275.54 | 1,342.44 ± 1,246.3 | 0.647* |
| Carboplatin, mg/m2 | 19.38 ± 111.87 | 0 ± 0 | 21.46 ± 117.54 | 0.296* |
| Total duration of anthracycline chemotherapy cycles, months | 4 (0 - 27) | 4 (1 - 20) | 4 (0 - 27) | 0.081† |
| Total (n = 341) | With AIC (n = 33) | Without AIC (n = 308) | P-value | |
|---|---|---|---|---|
| Statistical test used: ‡Chi-square/Fisher’s exact test. Data are expressed as frequency (%) or median (range). AIC: anthracycline-induced cardiotoxicity; LVEF: left ventricular ejection fraction. | ||||
| Mortality | 17 (4.99) | 2 (6.06) | 15 (4.87) | 0.674‡ |
| LVEF | ||||
| At first follow-up | 65.5 (55-92) | 57 (39 - 80) | 66 (55 - 92) | |
| At second follow-up (n = 178) | 64.4 (35 - 79) | 50.8 (35 - 67) | 65.1 (48.28 - 79) | |
| At third follow-up (n = 109) | 66 (27.6 - 86) | 53.3 (27.6 - 81) | 66.1 (55 - 86) | |
| Crude odds ratio (95% CI) | P-value | |
|---|---|---|
| Statistical test used: Firth logistic regression. BMI: body mass index; PTB: pulmonary tuberculosis; ACE: angiotensin converting enzyme; SGLT2: sodium-glucose cotransporter-2; HER2: human epidermal growth factor 2; LVEF: left ventricular ejection fraction; CI: confidence interval. | ||
| Age, years | 1.05 (1.01 - 1.09) | 0.005 |
| BMI, kg/m2 | ||
| Underweight (< 18.5) | 1.689 (0.37 - 7.75) | 0.500 |
| Normal (18.5 to < 23) | 1.0 (reference) | - |
| Overweight (23 to 27.5) | 0.537 (0.21 - 1.35) | 0.187 |
| Obese (> 27.5) | 0.891 (0.37 - 2.14) | 0.796 |
| Comorbidities | ||
| Any | 6.023 (2.49 - 14.58) | < 0.001 |
| Hypertension | 4.579 (2.16 - 9.7) | < 0.001 |
| Diabetes mellitus | 1.449 (0.61 - 3.44) | 0.401 |
| Dyslipidemia | 0.944 (0.3 - 3.02) | 0.922 |
| PTB | 2.547 (0.4 - 16.06) | 0.320 |
| Asthma | 1.01 (0.05 - 19.17) | 0.995 |
| Atrial fibrillation | 16.27 (2.08 - 127.29) | 0.008 |
| Coronary artery disease | 5.658 (0.72 - 44.23) | 0.099 |
| Chronic kidney disease | 5.658 (0.72 - 44.23) | 0.099 |
| Hyperthyroidism | 5.658 (0.72 - 44.23) | 0.099 |
| Hepatitis B | 1.83 (0.09 - 38.92) | 0.698 |
| Other | 1.615 (0.49 - 5.33) | 0.431 |
| Medications | ||
| Any medications | 2.728 (1.32 - 5.63) | 0.007 |
| Calcium channel blocker | 1.835 (0.8 - 4.23) | 0.154 |
| Angiotensin receptor blockers | 4.366 (2.04 - 9.36) | < 0.001 |
| Statin | 1.535 (0.58 - 4.09) | 0.391 |
| Oral hypoglycemic agent | 1.254 (0.44 - 3.6) | 0.674 |
| Beta-blocker | 5.174 (1.88 - 14.26) | 0.001 |
| ACE inhibitor | 7.765 (1.83 - 32.96) | 0.005 |
| Anti-hypertensive | 6.927 (1.31 - 36.58) | 0.023 |
| Anti-platelet | 3.123 (0.48 - 20.53) | 0.236 |
| Exogenous insulin | 0.824 (0.04 - 15.22) | 0.896 |
| Exogenous thyroid medication | 1.303 (0.07 - 25.77) | 0.862 |
| Anti-cholesterol medication | 1.303 (0.07 - 25.77) | 0.862 |
| Vitamins | 1.303 (0.07 - 25.77) | 0.862 |
| Cardiometabolic drugs | 5.658 (0.72 - 44.23) | 0.099 |
| Anti-arrhythmic medication | 9.462 (0.96 - 93.61) | 0.055 |
| Anti-thyroid medication | 1.83 (0.09 - 38.92) | 0.698 |
| Novel oral anticoagulant | 9.462 (0.96 - 93.61) | 0.055 |
| Nutritional supplements | 9.462 (0.96 - 93.61) | 0.055 |
| SGLT2 inhibitors | 9.462 (0.96 - 93.61) | 0.055 |
| Anti-asthma medications | 3.06 (0.12 - 76.61) | 0.496 |
| Oral antimetabolite | 3.06 (0.12 - 76.61) | 0.496 |
| Anti-tuberculosis medications | 3.06 (0.12 - 76.61) | 0.496 |
| Low molecular weight heparin | 3.06 (0.12 - 76.61) | 0.496 |
| Concurrent chemotherapeutic agents | ||
| Any | 0.061 (0.02 - 0.15) | < 0.001 |
| Cyclophosphamide | 0.136 (0.06 - 0.29) | < 0.001 |
| Taxanes | 0.277 (0.13 - 0.57) | 0.001 |
| HER-2 inhibitors | 0.494 (0.21 - 1.15) | 0.101 |
| 5-fluorouracil | 0.22 (0.04 - 1.16) | 0.075 |
| Carboplatin | 0.303 (0.02 - 5.2) | 0.410 |
| Monoclonal antibodies | 1.83 (0.09 - 38.92) | 0.698 |
| Eribulin | 9.462 (0.96 - 93.61) | 0.055 |
| Vinorelbine | 28.477 (1.14 - 713.39) | 0.042 |
| Radiation therapy | ||
| No radiotherapy | 1.0 (reference) | - |
| Left breast radiotherapy | 0.598 (0.03 - 10.73) | 0.727 |
| Right chest wall radiotherapy | 1.281 (0.32 - 5.08) | 0.724 |
| Mediastinal radiotherapy | 1.17 (0.2 - 6.68) | 0.860 |
| Baseline LVEF | 0.929 (0.86 - 1) | 0.051 |
| Chemotherapeutic agents dosage | ||
| 5-fluorouracil, mg/m2 | 1 (0.99 - 1.0) | 0.354 |
| Epirubicin, mg/m2 | 0.991 (0.98 - 1) | 0.050 |
| Doxorubicin, mg/m2 | 1.002 (1 - 1) | 0.076 |
| Idarubicin, mg/m2 | 1.001 (1 - 1) | 0.650 |
| Daunorubicin, mg/m2 | - | - |
| Trastuzumab, mg | 1 (1 - 1) | 0.988 |
| Docetaxel, mg/m2 | 0.999 (1 - 1) | 0.656 |
| Paclitaxel, mg/m2 | 1 (0.99 - 1.0) | 0.844 |
| Cyclophosphamide, mg | 1 (0.99 - 1.0) | 0.574 |
| Carboplatin, mg/m2 | 0.999 (0.99 - 1) | 0.635 |
| Total duration of anthracycline chemotherapy cycles, months | 1.04 (0.97 - 1.11) | 0.239 |
| Adjusted odds ratio (95% CI) | P-value | |
|---|---|---|
| Cox-Snell R2: 0.146; P < 0.001. LVEF: left ventricular ejection fraction; CI: confidence interval. | ||
| Age ≥ 65 years old | 0.879 (0.28 - 2.8) | 0.827 |
| Any comorbidity | 12.369 (3.58 - 42.74) | < 0.001 |
| Any medications | 0.369 (0.13 - 1.07) | 0.065 |
| Presence of any concurrent chemotherapy | 0.07 (0.03 - 0.19) | < 0.001 |
| Presence of concomitant radiation therapy | 0.691 (0.17 - 2.84) | 0.608 |
| Baseline LVEF | 0.954 (0.88 - 1.03) | 0.245 |
| Doxorubicin dosage > 250, mg/m2 | 1.497 (0.33 - 6.88) | 0.604 |
| Epirubicin dosage > 600, mg/m2 | 0.415 (0.02 - 8.2) | 0.563 |