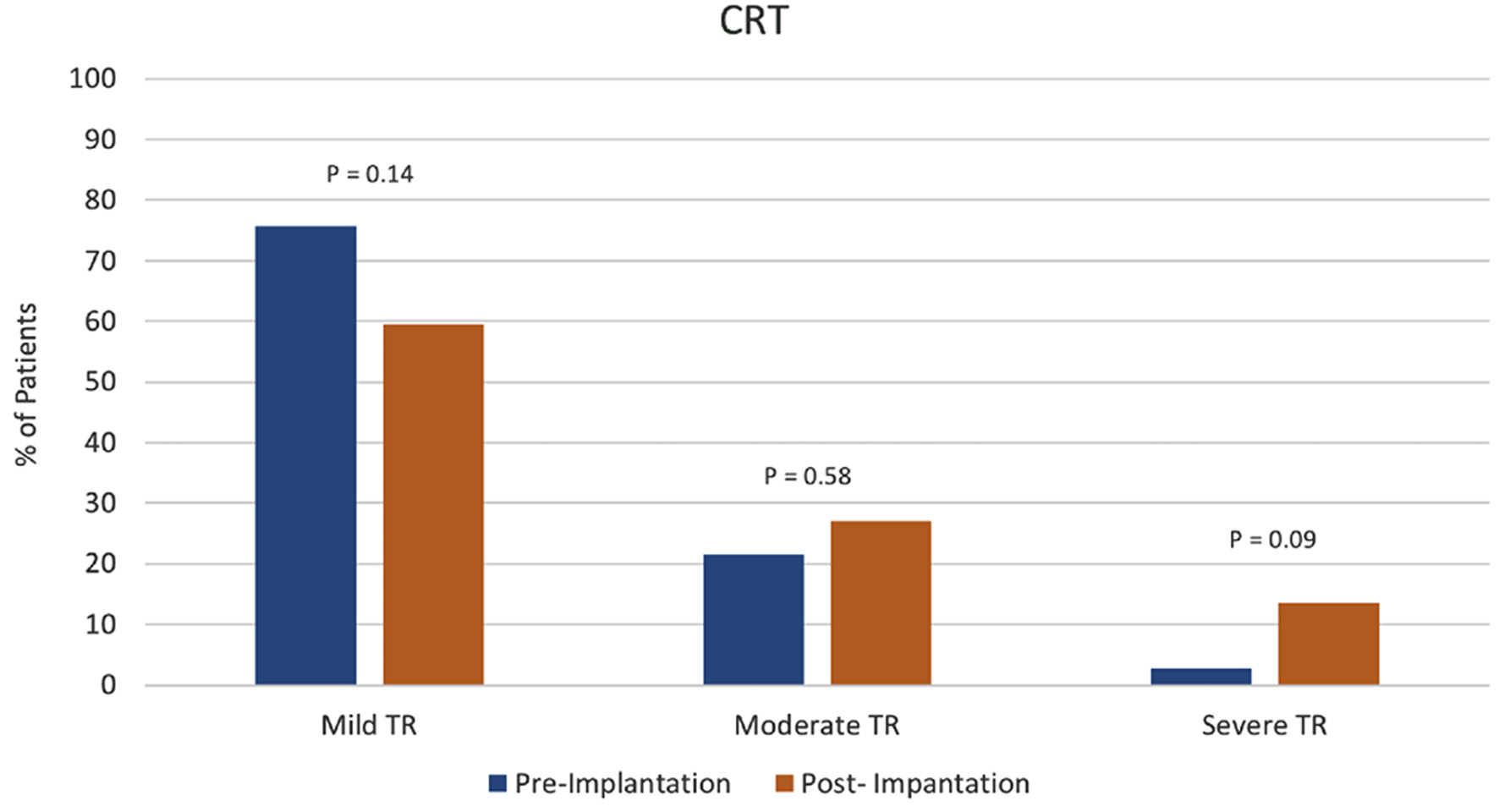
Figure 1. Cardiac resynchronization therapy (CRT) group tricuspid regurgitation (TR) severity pre- vs. post-device implantation.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://www.cardiologyres.org |
Original Article
Volume 13, Number 3, June 2022, pages 128-134
Comparison of Tricuspid Regurgitation Severity Between Cardiac Resynchronization Therapy Versus Right Ventricular Pacing in Patients With Chronic Obstructive Pulmonary Disease
Figures

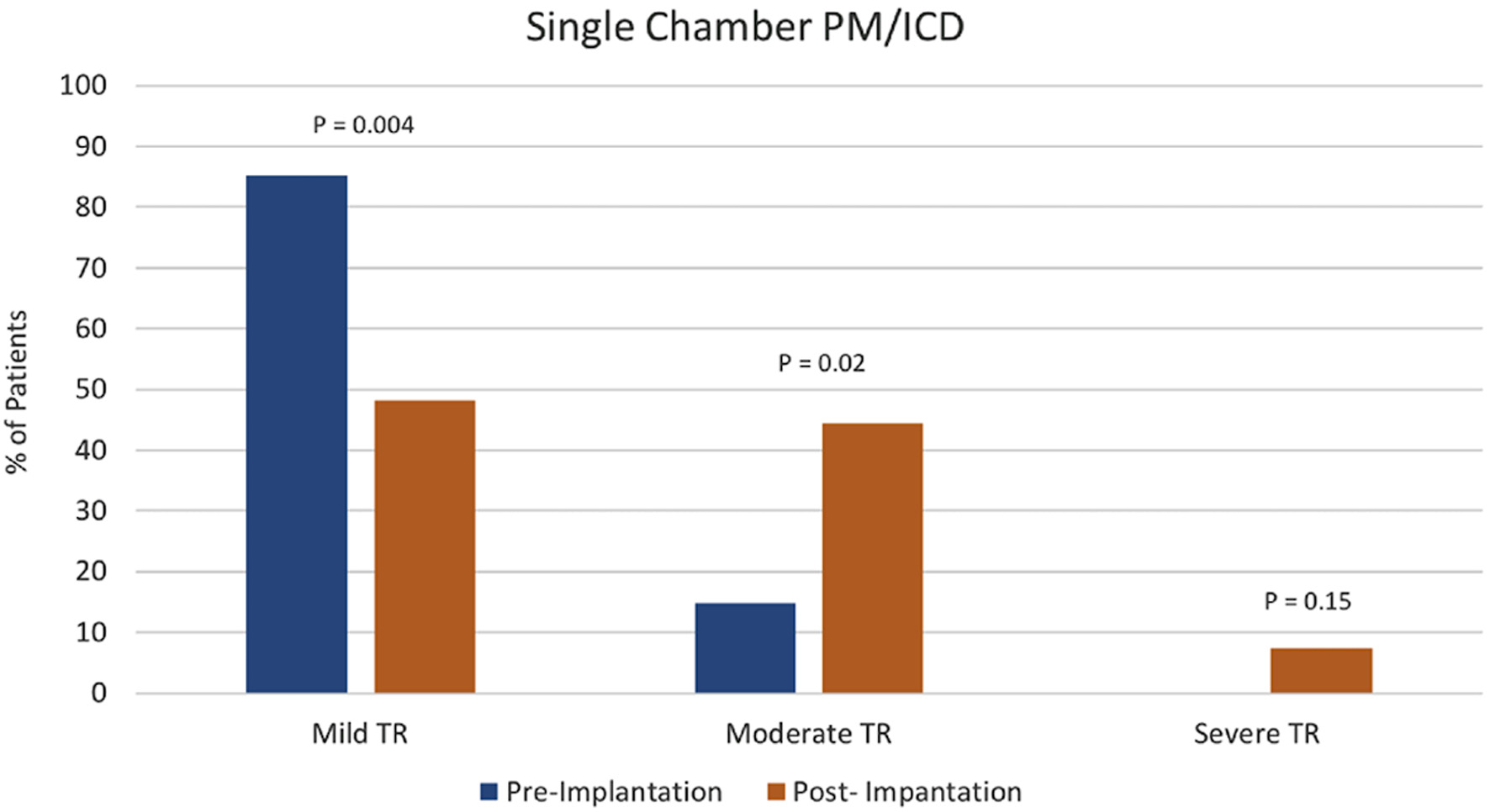
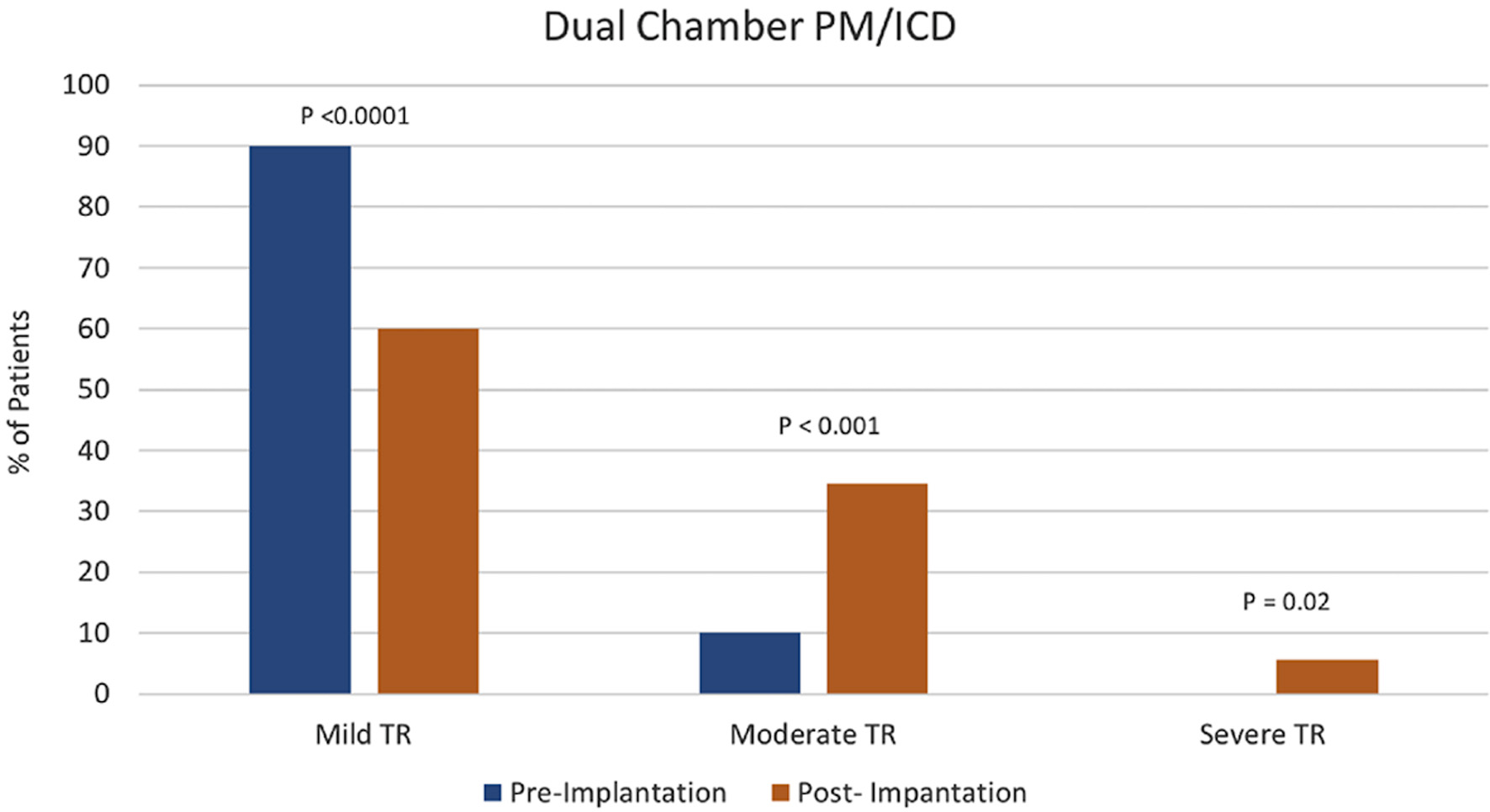
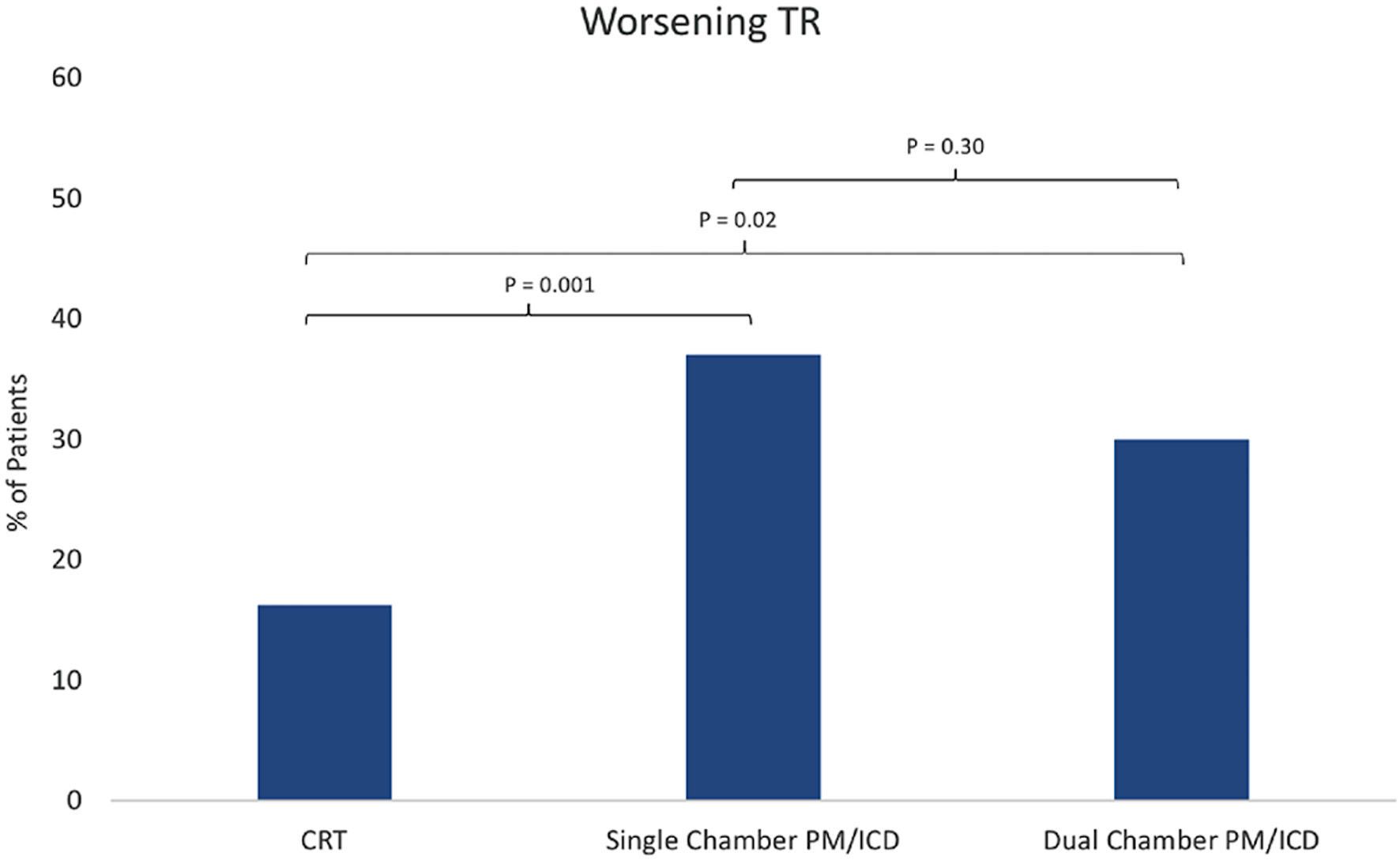
Tables
| Characteristics | CRT (n = 37) | Single-chamber PPM/ICD (n = 27) | Dual-chamber PPM/ICD (n=90) | P value |
|---|---|---|---|---|
| Variables are expressed as no (%) or mean ± standard deviation. P < 0.05 indicates difference between the groups is statistically significant. CRT: cardiac resynchronization therapy; PPM: permanent pacemaker; ICD: implantable cardioverter-defibrillator; CRT-D: cardiac resynchronization therapy defibrillator; CRT-P: cardiac resynchronization therapy pacemaker; ICD-S: implantable cardioverter-defibrillator single-chamber; PPM-S: permanent pacemaker single-chamber; PPM-D: permanent pacemaker dual-chamber; ICD + PPM-D: implantable cardioverter-defibrillator with permanent pacemaker dual-chamber; RVSP: right ventricular systolic pressure. | ||||
| Age (years) | 70.22 ± 9.37 | 70.07 ± 10.06 | 72.79 ± 11.15 | 0.32 |
| Male sex | 23 (62.16%) | 16 (59.26%) | 46 (51.11%) | 0.46 |
| Device subtypes | ||||
| CRT-D | 19 (51.35%) | |||
| CRT-P | 18 (49.65%) | |||
| ICD-S | 8 (29.63%) | |||
| PPM-S | 19 (70.37%) | |||
| PPM-D | 80 (88.89%) | |||
| ICD + PPM-D | 10 (11.11%) | |||
| Ejection fraction (%) | ||||
| Pre-device implantation | 33.53±10.86% | 45.56±13.68% | 51.29 ± 11.20 | < 0.0001 |
| Post-device Implantation | 46.53±13.14% | 49.26±11.82% | 50.69±13.34% | 0.41 |
| RVSP (mm Hg) | ||||
| Pre-device implantation | 31.82 ± 8.42 | 34.65 ± 7.22 | 35.73 ± 14 | 0.42 |
| Post-device implantation | 40.54 ± 16.07 | 46.41 ± 16.31 | 39.07 ± 13.42 | 0.11 |
| Degree of TR | CRT (n = 37) | Single-chamber device (n = 27) | Dual-chamber device (n = 90) |
|---|---|---|---|
| TR: tricuspid regurgitation; CRT: cardiac resynchronization therapy. | |||
| Mild | 28 | 23 | 81 |
| Moderate | 8 | 4 | 9 |
| Severe | 1 | 0 | 0 |